Mesoporous Ru-MIL-125-NH2 catalyst prepared from supercutical fluid
A ru-mil-125-nh2, supercritical fluid technology, applied in the field of materials, can solve the problem of small pore size and achieve the effect of improving reactivity and visible light absorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The high mesoporous Ru-MIL-125-NH of the present embodiment 2 The synthesis method is as follows:
[0033] Synthesis of highly mesoporous Ru-MIL-125-NH 2 The metal salt precursor used is RuCl 3 ·xH 2 O, MOFs material is MIL-125-NH 2 .
[0034] MIL-125-NH 2 with RuCl 3 ·xH 2 O was added to the reaction kettle according to the mass ratio of 100:5, stirred at room temperature until uniformly mixed, then filled with carbon dioxide at a certain pressure (5.58MPa), reacted for 5 hours at a reaction temperature of 200°C, and cooled to room temperature , remove the carbon dioxide in the reactor by pressure relief, centrifuge to obtain a solid precipitate, wash the obtained precipitate with DMF and methanol, and then place it in a vacuum drying oven to remove the remaining solvent molecules, the drying temperature is 80 ° C, Ru-MIL-125-NH was obtained after drying for 12 hours 2 .
[0035] MIL-125-NH 2 And the obtained high mesoporous Ru-MIL-125-NH of this embodiment ...
Embodiment 2
[0039] Mesoporous Ru-MIL-125-NH of the present embodiment 2 The synthesis method is as follows:
[0040] Synthesis of Mesoporous Ru-MIL-125-NH 2 The metal salt precursor used is RuCl3 ·xH 2 O, MOFs material is MIL-125-NH 2 .
[0041] MIL-125-NH 2 with RuCl 3 ·xH 2 O is added to the reaction kettle according to the mass ratio of 100:5, stirred at room temperature until uniformly mixed, then filled with carbon dioxide at a certain pressure (5.58MPa), and reacted for 1 hour at a reaction temperature of 200°C, then cooled to room temperature , remove the carbon dioxide in the reactor by pressure relief, centrifuge to obtain a solid precipitate, wash the obtained precipitate with DMF and methanol, and then place it in a vacuum drying oven to remove the remaining solvent molecules, the drying temperature is 80 ° C, Ru-MIL-125-NH was obtained after drying for 12 hours 2 .
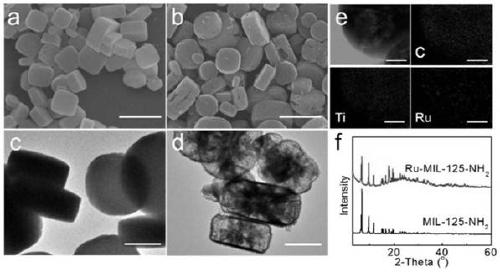
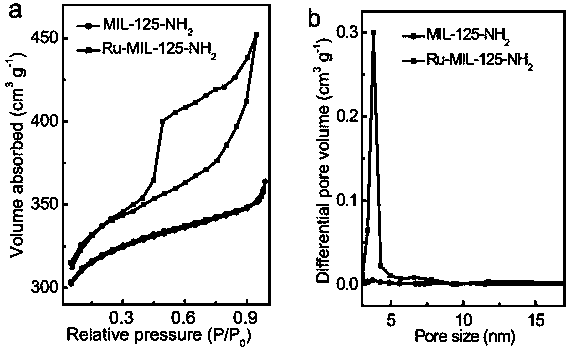
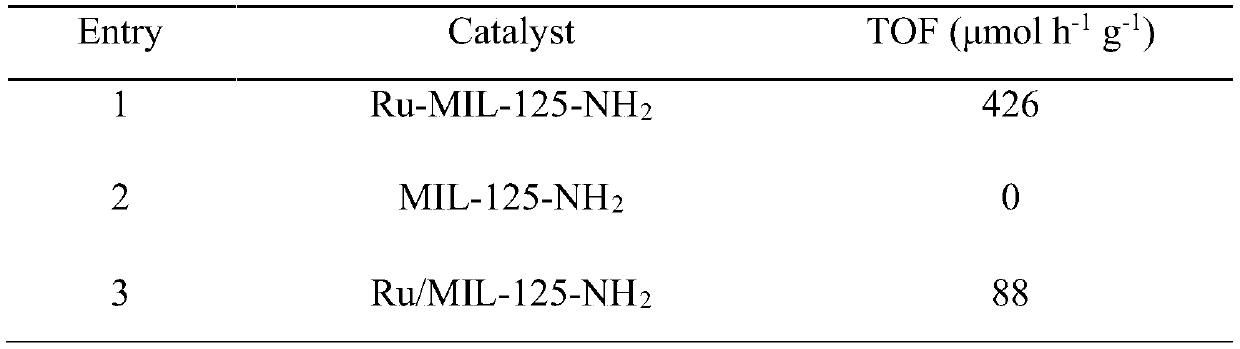
[0042] The resulting Ru-MIL-125-NH 2 Scanning, transmission electron microscopy, X-ray diffraction (X...
Embodiment 4
[0044] Mesoporous Ru-MIL-125-NH of the present embodiment 2 The synthesis method is as follows:
[0045] Synthesis of Mesoporous Ru-MIL-125-NH 2 The metal salt precursor used is RuCl 3 ·xH 2 O, MOFs material is MIL-125-NH 2 .
[0046] MIL-125-NH 2 with RuCl 3 ·xH 2 O was added to the reaction kettle according to the mass ratio of 100:5, stirred at room temperature until uniformly mixed, then filled with carbon dioxide at a certain pressure (5.58MPa), reacted for 3 hours at a reaction temperature of 200°C, and cooled to room temperature , remove the carbon dioxide in the reactor by pressure relief, centrifuge to obtain a solid precipitate, wash the obtained precipitate with DMF and methanol, and then place it in a vacuum drying oven to remove the remaining solvent molecules, the drying temperature is 80 ° C, Ru-MIL-125-NH was obtained after drying for 12 hours 2 .
[0047] The resulting Ru-MIL-125-NH 2 Scanning, transmission electron microscopy, X-ray diffraction (XR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com