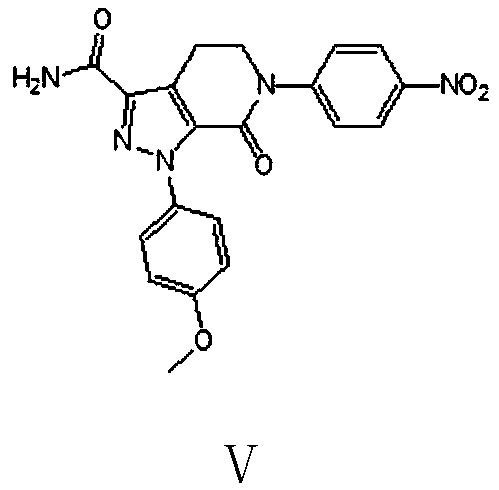
Preparation method of apixaban
A technology for apixaban and its compounds, which is applied in the field of preparation of apixaban, and can solve problems such as cumbersome operation, large amount of waste water, and long steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
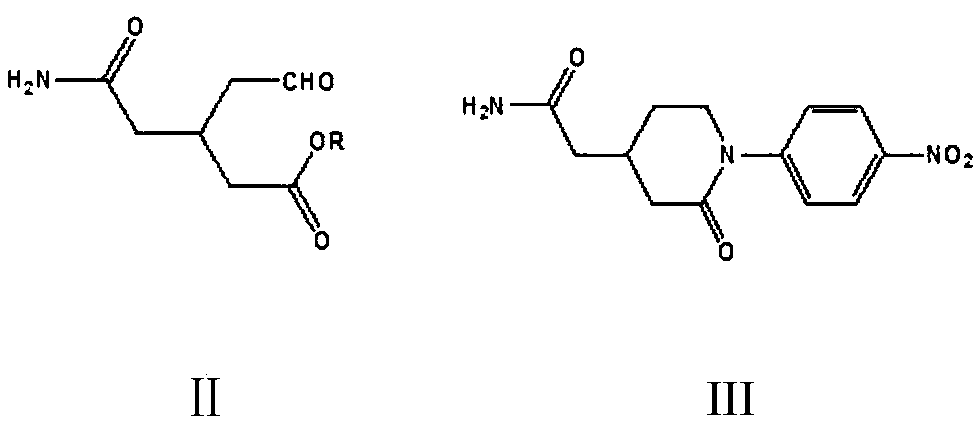
[0072] Embodiment 1: Preparation of 1-(4-nitrophenyl)piperidin-2-one-4-acetamide (III)
[0073] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser and dropping funnel, add 50 gram methanol, 60 gram toluene, 13.8 gram (0.1 mole) 4-nitroaniline, 4.5 gram sodium borohydride, 19.0 gram (0.1 mole) of 3-formylmethyl n-glutaric acid monomethyl ester monoamide (II), stirred and reacted at 60 to 65°C for 5 hours, and stirred at 95 to 100°C for 5 hours, while distilling and recovering methanol. Cool to 20 to 25°C, use 20wt% ammonium chloride aqueous solution to acidify the pH value of the system to 4.0-4.5, add 100 grams of dichloromethane, separate layers, extract the water layer with dichloromethane 3 times, 20 grams each time, and combine the organic phases , Dichloromethane and toluene were recovered by distillation to obtain 24.6 grams of 1-(4-nitrophenyl)piperidin-2-one-4-acetamide, the yield was 88.8%, and the liquid phase purit...
Embodiment 2
[0074] Example 2: Preparation of 1-(4-nitrophenyl)piperidin-2-one-4-acetamide (III)
[0075] In the 500 milliliter four-neck flask that is connected with stirring, thermometer, reflux condenser and dropping funnel, add 50 grams of tetrahydrofuran, 60 grams of toluene, 13.8 grams (0.1 moles) of 4-nitroaniline, 30.5 grams of triacetoxyboron Sodium hydride, 19.0 g (0.1 mole) of 3-formylmethyl n-glutaric acid monomethyl ester monoamide (II), stirred at 50 to 55°C for 6 hours, stirred at 95 to 100°C for 5 hours, and distilled and recovered tetrahydrofuran at the same time . Cool to 20 to 25°C, use 20wt% ammonium chloride aqueous solution to acidify the pH value of the system to 4.0-4.5, add 100 grams of dichloromethane, separate layers, extract the water layer with dichloromethane 3 times, 20 grams each time, and combine the organic phases , dichloromethane, tetrahydrofuran and toluene were recovered by distillation to obtain 24.9 g of 1-(4-nitrophenyl)piperidin-2-one-4-acetamide,...
Embodiment 3
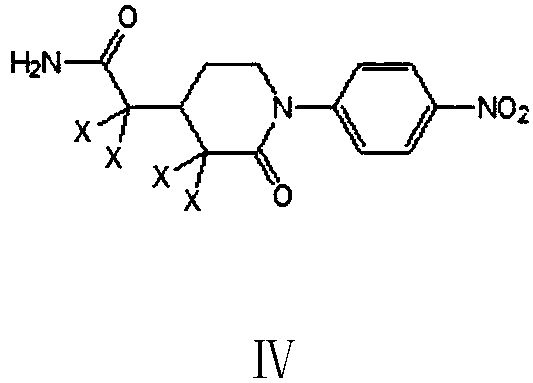
[0076] Example 3: Preparation of 1-(4-nitrophenyl)piperidin-2-one-3,3-dichloro-4-dichloroacetamide (Ⅳ1)
[0077] In the 500 milliliter four-necked flask that is connected with stirring, thermometer, reflux condenser and dropping funnel, add 220 grams of 1,2-dichloroethane, 13.9 grams (0.1 moles) of 1-(4- Nitrophenyl)piperidin-2-one-4-acetamide (III), 52.5 g of phosphorus pentachloride, stirred and reacted between 50 and 55° C. for 5 hours. Cool to 20 to 25°C, slowly add the resulting reaction mixture to 100 g of 1,2-dichloroethane and 300 g of crushed ice, and keep stirring, separate layers, and use 1,2-dichloroethane for the water layer Extract 3 times, 20 grams each time, combine the organic phases, and distill and recover 1,2-dichloroethane to obtain 21.2 grams of light brown viscous liquid 1-(4-nitrophenyl)piperidin-2-one-3 , 3-dichloro-4-dichloroacetamide was directly used in the next step (Example 5).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com