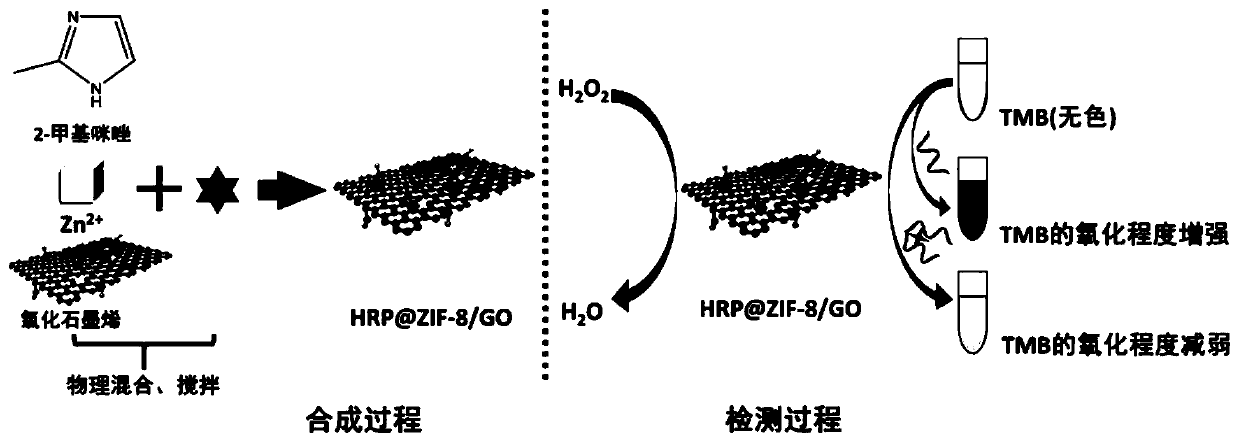
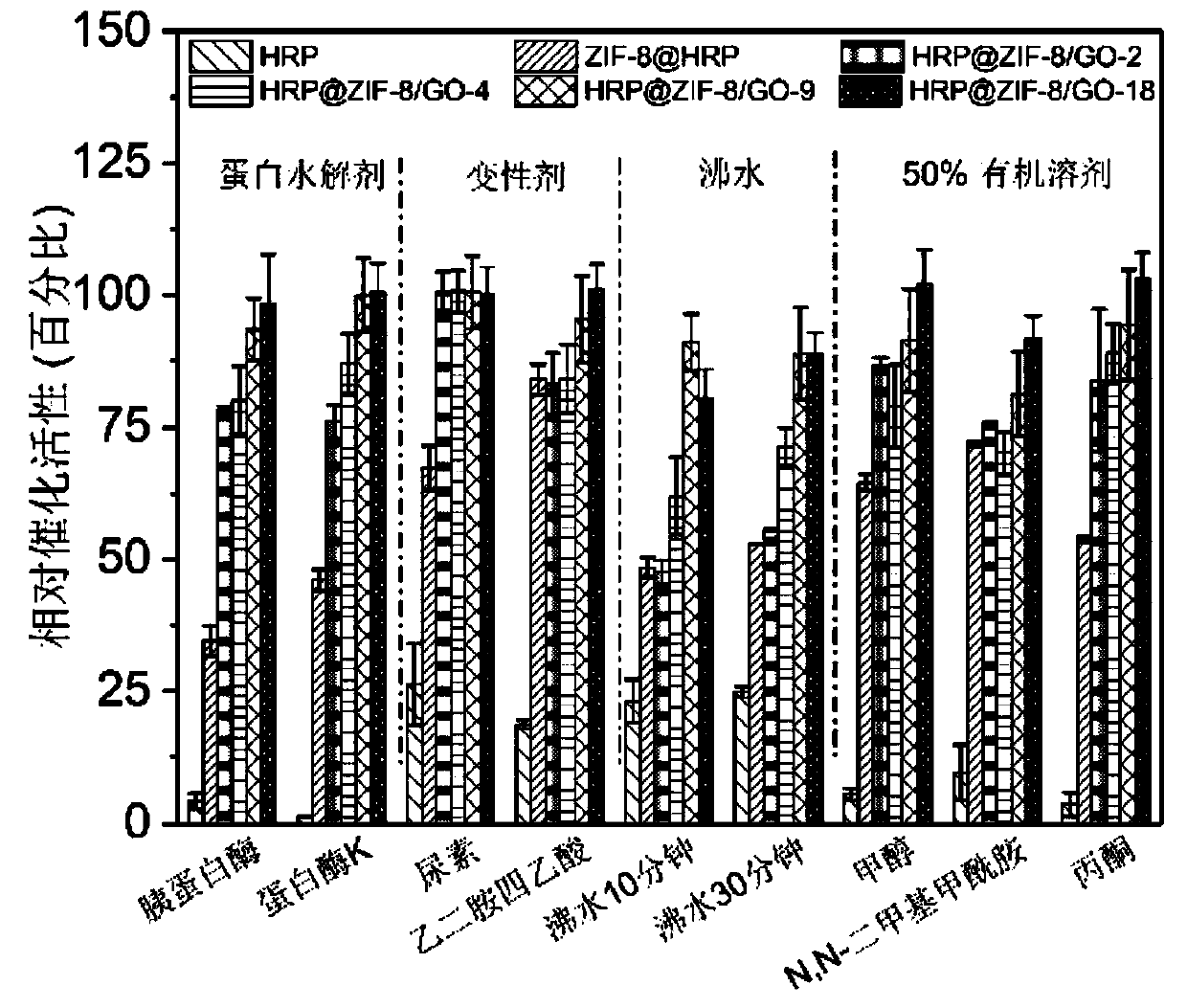
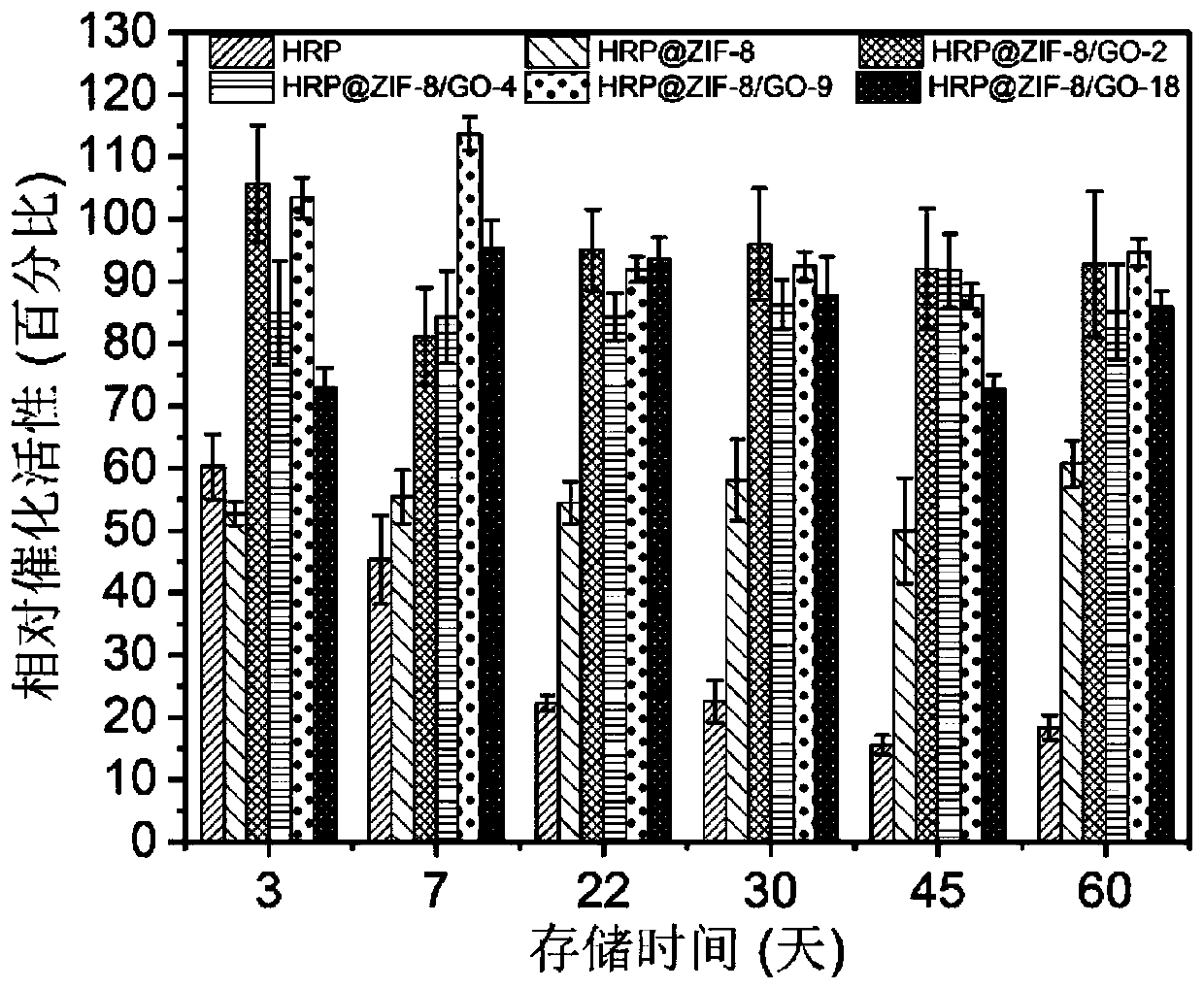
Immobilized enzyme method for improving stability of horseradish peroxidase and application
A technology of horseradish peroxidase and immobilized enzyme, applied in the directions of enzyme stabilization, oxidoreductase, biochemical equipment and methods, etc., can solve problems such as insufficient stability of natural enzymes, and achieve improved storage stability and protection performance. The effect of enhancing, enhancing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Configure the determination of kanamycin content in water samples:
[0028] (1) Preparation of graphene oxide:
[0029] Graphene oxide was prepared using a modified Hummers chemistry. The specific steps are as follows: Weigh 1.0g of graphite powder and slowly add it to 23mL of concentrated sulfuric acid. After fully stirring, slowly add 3g of KMnO 4 , slowly added while fully stirring, and then continuously ultrasonicated for 7 hours to obtain a dark brown solution, then slowly added 46mL of deionized water, heated and boiled for 15min, then added 140mL of high-purity water and 10mL of hydrogen peroxide to terminate the reaction, and obtained bright yellow graphene oxide aqueous solution. After centrifugation, use 5% dilute hydrochloric acid 8000r / min centrifugal washing 2 times to remove impurities, and then use high-purity water 8000r / min centrifugal washing 3 times to remove impurities. After washing, the purified graphite oxide was taken out, put into a dialysis ...
Embodiment 2
[0040] Configure the determination of kanamycin content in water samples:
[0041] (1) Preparation of graphene oxide:
[0042] Graphene oxide was prepared using a modified Hummers chemistry. The specific steps are as follows: Weigh 1.0g of graphite powder and slowly add it to 23mL of concentrated sulfuric acid. After fully stirring, slowly add 3g of KMnO 4 , slowly added while fully stirring, and then continuously ultrasonicated for 6 hours to obtain a dark brown solution, then slowly added 46mL of deionized water, heated and boiled for 15min, then added 140mL of high-purity water and 10mL of hydrogen peroxide to terminate the reaction, and obtained bright yellow graphene oxide aqueous solution. After centrifugation, use 5% dilute hydrochloric acid 10000r / min centrifugal washing 2 times to remove impurities, and then use high-purity water 10000r / min centrifugal washing 5 times to remove impurities. After washing, the purified graphite oxide was taken out, put into a dialysi...
Embodiment 3
[0053] Determination of Kanamycin Content in Tap Water Samples:
[0054] (1) Preparation of graphene oxide:
[0055] Graphene oxide was prepared using a modified Hummers chemistry. The specific steps are as follows: Weigh 1.0g of graphite powder and slowly add it to 23mL of concentrated sulfuric acid. After fully stirring, slowly add 3g of KMnO 4 , slowly added while fully stirring, and then continuously ultrasonicated for 5 hours to obtain a dark brown solution, then slowly added 46mL of deionized water, heated and boiled for 15min, then added 140mL of high-purity water and 10mL of hydrogen peroxide to terminate the reaction, and obtained bright yellow graphene oxide aqueous solution. After centrifugation, use 5% dilute hydrochloric acid 10000r / min centrifugal washing 2 times to remove impurities, and then use high-purity water 10000r / min centrifugal washing 5 times to remove impurities. After washing, the purified graphite oxide was taken out, put into a dialysis bag (MW=...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com