Oral sustained-release preparation of hydroxypiperidone
A technology for hydroxypiperidine and sustained-release preparations, which is applied in the field of medicine, can solve the problems of long interval between taking medicines at night, cannot maintain blood drug concentration, and treat blind spots, etc., and can meet the slow-release release effect, reduce the number of times of taking medicines, and be convenient to take. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
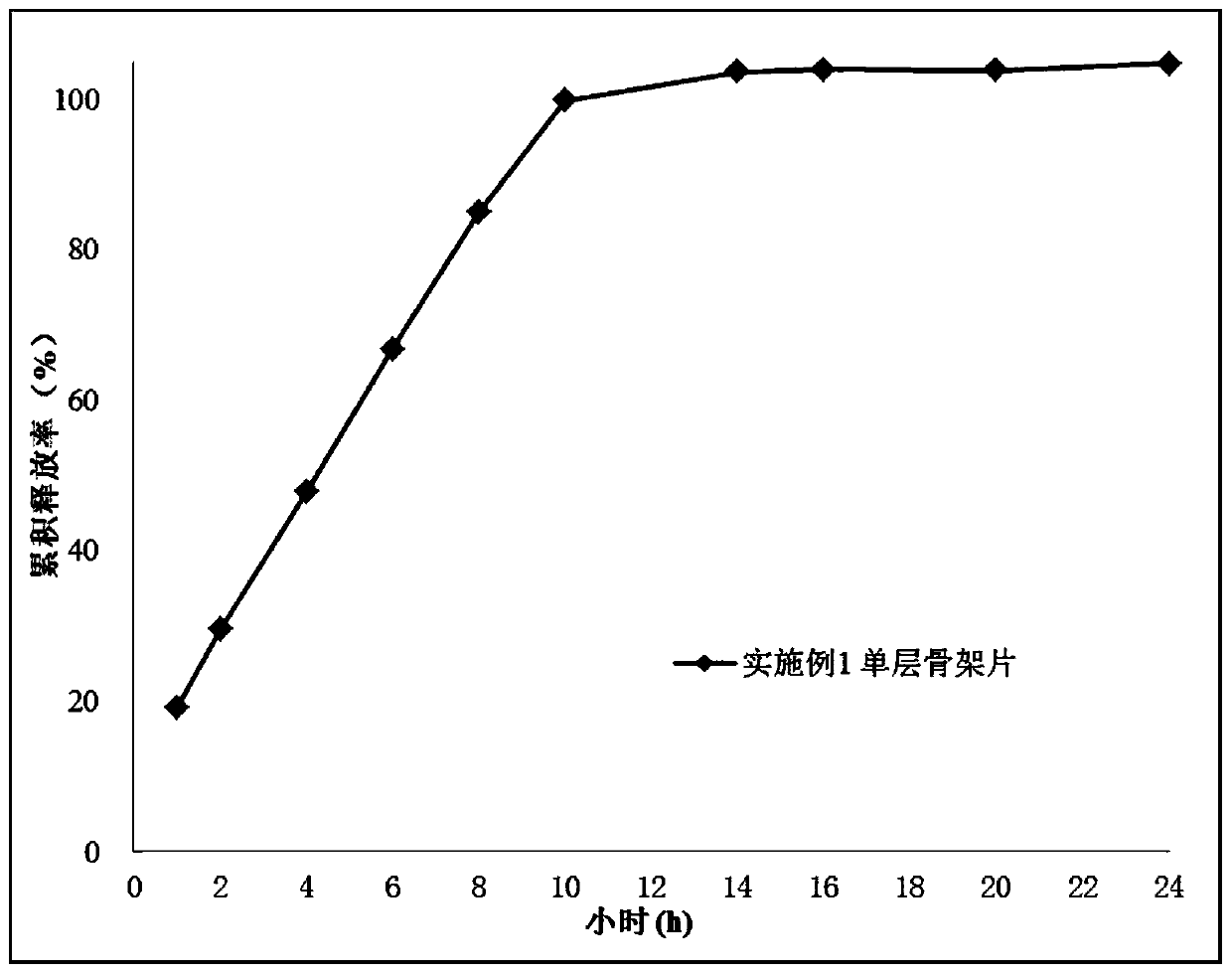
[0071] Embodiment 1 (single-layer matrix sustained-release tablet)
[0072] This embodiment is a common single-layer matrix sustained-release tablet of piperaperone, and the composition and content of its drug layer are as shown in Table 1:
[0073] Table 1
[0074]
[0075]
[0076] Preparation Process:
[0077] 1. Take the prescribed amount of piperidone hydrochloride, hydroxypropyl methylcellulose, sodium carmellose, microcrystalline cellulose and pregelatinized starch and mix evenly;
[0078] 2. Use 5% PVPK30 aqueous solution to make soft materials, and pass through a 2.0mm screen to make wet granules;
[0079] 3. The obtained wet granules are dried in a fluidized bed until the water content is less than 2%.
[0080] 4. After passing the dry granules through a 1.2mm sieve for granulation, mix them with the prescribed amount of magnesium stearate, and compress them into tablets. The theoretical tablet weight is 150mg.
[0081] Release test, adopt Chinese Pharmacop...
Embodiment 2
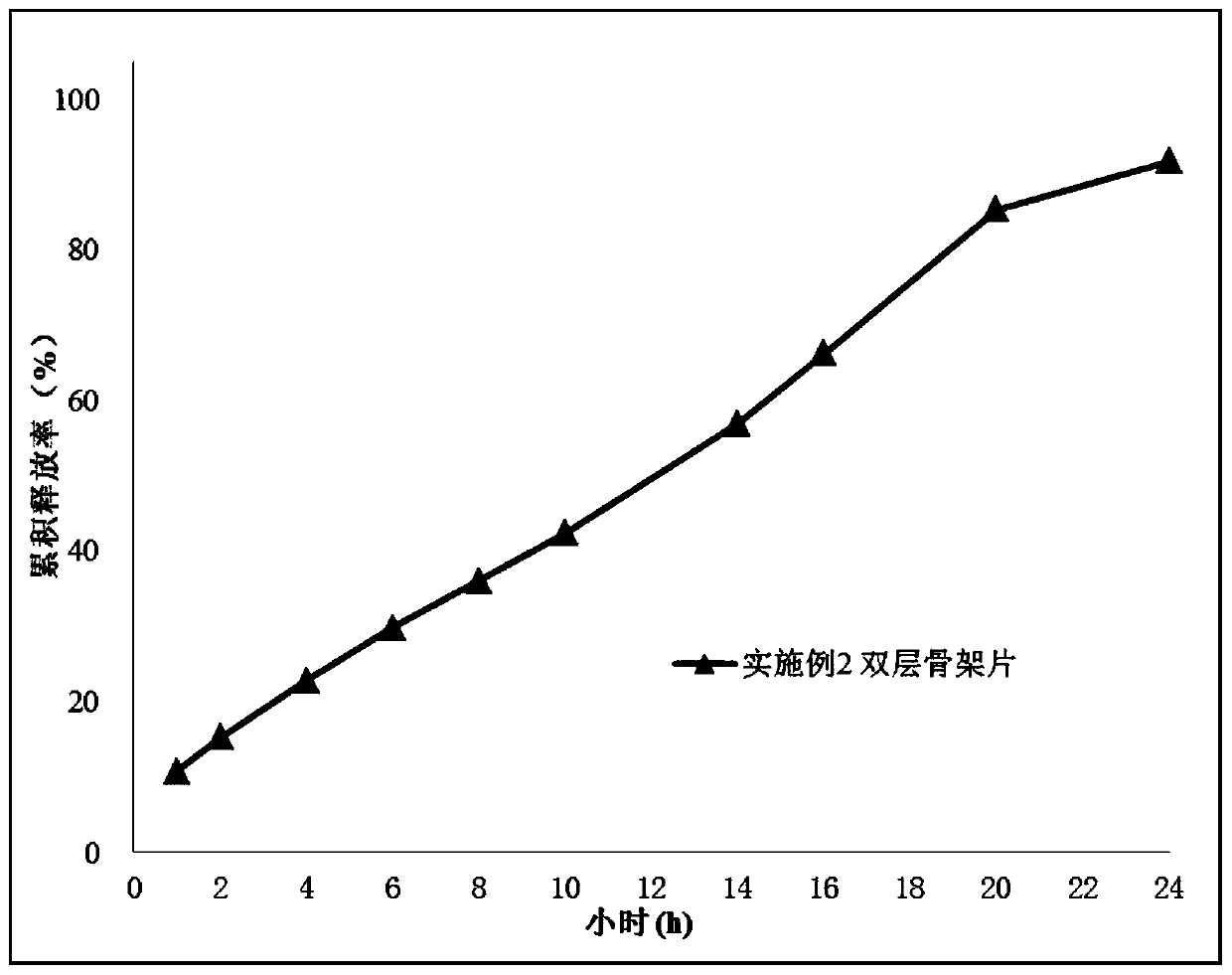
[0083] Embodiment 2 (double-layer matrix sustained-release tablet)
[0084] The present embodiment is a double-layer matrix sustained-release tablet of piperidone, and the composition and content of the drug layer are as shown in Table 2:
[0085] Table 2
[0086]
[0087]
[0088] The composition and content of the barrier layer of the dipiprone double-layer matrix sustained-release tablet are as shown in Table 3:
[0089] table 3
[0090] components Dosage (mg) lactose monohydrate 47.7 yellow iron oxide 0.3 Hydroxypropyl Methyl Cellulose (HPMC, K4M) 60 Glyceryl behenate 37.5 Polyvinylpyrrolidone (PVP, K30) 3 Magnesium stearate 1.5 Total 150
[0091] The preparation process of the drug layer of the bilayer matrix sustained-release tablet of piperidone:
[0092] 1. Take the prescribed amount of piperidone hydrochloride, hydroxypropyl methylcellulose, sodium carmellose, lactose monohydrate and glyceryl behenate ...
Embodiment 3
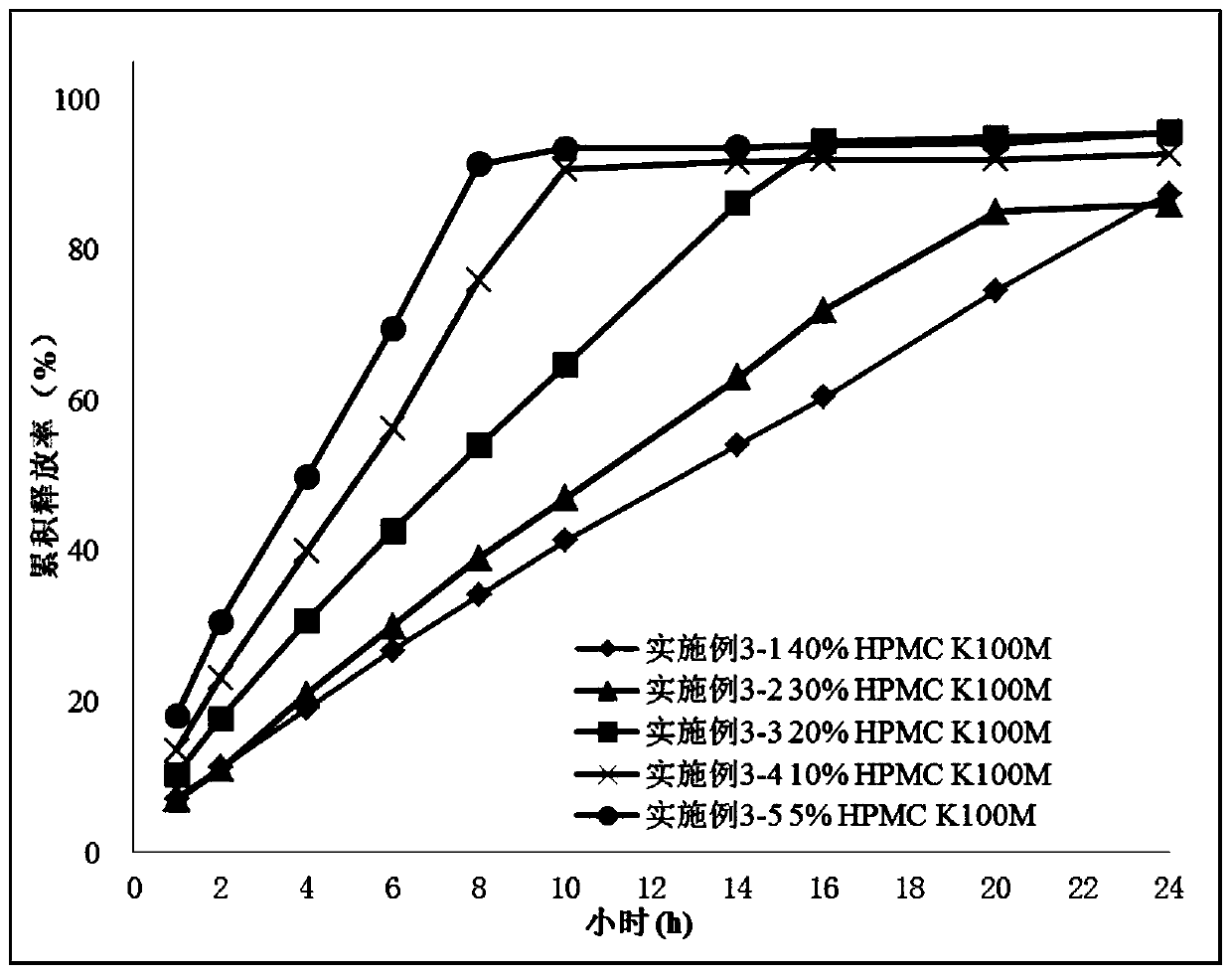
[0105] Embodiment 3 (three-layer matrix sustained-release tablet)
[0106] This example is a three-layer matrix sustained-release tablet of piperaperone with different hydroxypropylmethylcellulose contents, and the composition and content of the drug layer are as shown in Table 4:
[0107] Table 4
[0108]
[0109] The preparation process of the drug layer of the three-layer matrix sustained-release tablet of piperidone: the same as in Example 2.
[0110] The composition and preparation process of the barrier layer of the three-layer matrix sustained-release tablet of piperaperone: the same as in Example 2.
[0111] Tablets:
[0112] The above-prepared drug layer granules and barrier layer granules were pressed into a three-layer tablet. The first layer was composed of a 150 mg barrier layer, the second layer was composed of a 150 mg drug layer, and contained 20 mg of piperapidone, and the third layer was composed of 150 mg of barrier layer composition.
[0113] Coating...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com