A kind of immunoenhancing composition, preparation method and application thereof
A technology of immune enhancement and composition, applied in the field of medical biomaterials, can solve the problems of local abscess, severe reaction, short duration, etc., and achieve the effect of enhancing immune response, controllable degradation process, and long action period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The novel immune-enhancing composition of the present embodiment is prepared through the following steps:
[0046] (1) Organizational preprocessing:
[0047] The small intestinal submucosa tissue material was divided into 10cm wide and 15cm long predetermined size, the lymphoid tissue was removed, rinsed three times with tap water, and then rinsed with purified water until the surface was free of stains, and the water was drained.
[0048] (2) Virus inactivation:
[0049] Use peracetic acid-ethanol solution to soak small intestinal submucosa tissue material for virus inactivation, and this process can be carried out in a stainless steel barrel; the concentration of peracetic acid in the peracetic acid-ethanol solution is 1% (volume percent), the concentration of ethanol is 24% (volume percentage), the ratio (volume ratio) of peracetic acid-ethanol solution to small intestinal submucosa tissue material is 9:1, the inactivation time is 2 hours, and the temperature range ...
Embodiment 2
[0060] The performance test is carried out to the sample in Example 1, and the test items and results are as follows:
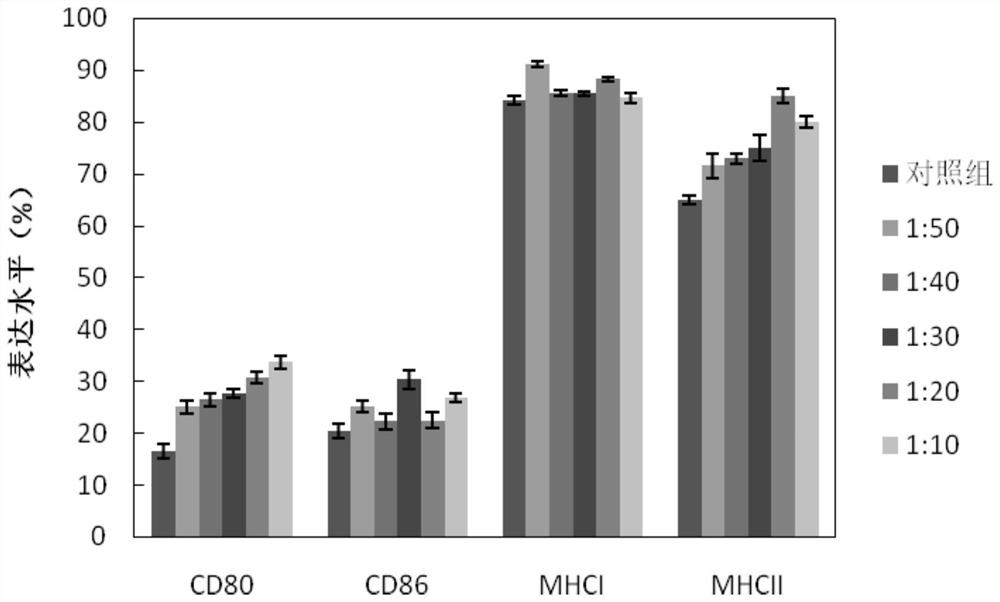
[0061] 1) Identification of collagen subtypes: I, III, IV and VI collagens were detected by immunohistochemical staining, 3 μm thick serial sections, dewaxed in xylene, and dehydrated in graded ethanol. Move the slices into a rice cooker water bath (containing 0.01 mol / L, pH 6.0 trisodium citrate buffer), keep the temperature at 95-100 °C, cook for 20 min, carry out antigen retrieval, and then naturally cool at room temperature after taking out. Wash with phosphate buffered saline (PBS), 5 min×3 times. Two-step immunohistochemistry: primary antibodies of collagen I, III, IV and VI were added dropwise at a concentration of 1:100, incubated at 4°C overnight at room temperature for 60 min, and washed 3 times with PBS. Add the Envision reaction solution dropwise and incubate at room temperature for 30 min. Washed 3 times with PBS. 0.05% 3,3-diaminobenzidine + ...
Embodiment 3
[0072] The biocompatibility test was carried out on the sample in Example 1, and the test items included: pyrogen, cytotoxicity, delayed type hypersensitivity reaction, intradermal reaction, acute systemic toxicity, Ames test, mouse lymphoma cell mutation test, chromosomal aberration , implantation, subchronic toxicity
[0073] 1) Pyrogen
[0074] Prepare the test solution according to the mass ratio of 1:5 leaching medium, 37±1℃, 72±2hr, leaching medium: physiological saline. According to the method specified in GB / T 14233.2-2005, the product has no pyrogen reaction.
[0075] 2) Cytotoxicity
[0076] Prepare the test solution according to the mass ratio of 1:5 leaching medium, 37±1℃, 24±2hr, leaching medium: MEM medium containing serum. Take the test solution and conduct the test according to the test method specified in GB / T16886.5-2003. The result shows that the cytotoxicity of the product is not greater than grade 1.
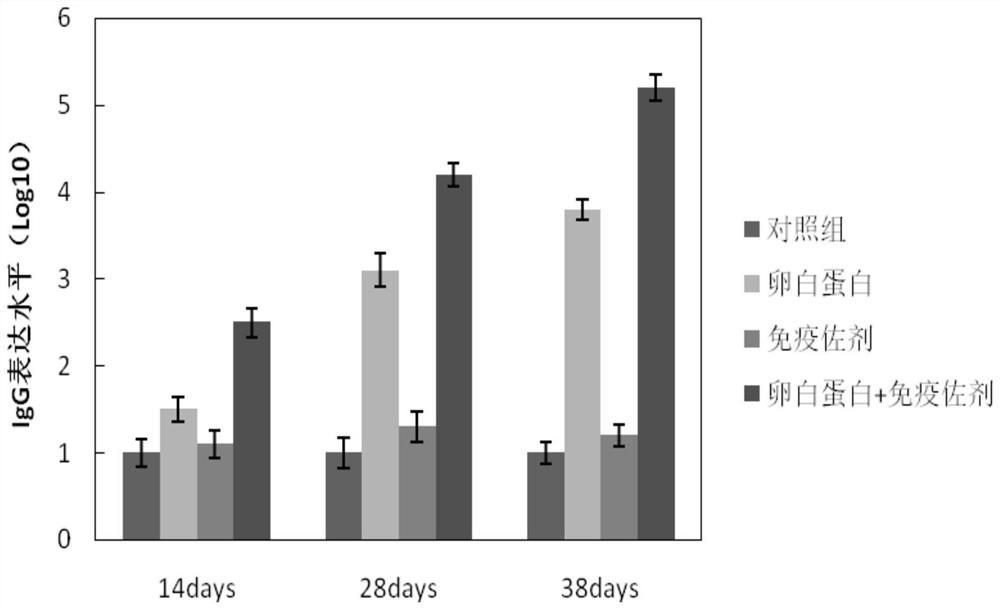
[0077] 3) Delayed type hypersensitivity
[0078] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com