Bisindole alkaloid compound or pharmaceutically acceptable salt thereof, and preparation method and application thereof
A technology of bis-indole alkaloids and indole alkaloids, which can be used in organic chemistry, drug combinations, and pharmaceutical formulations, and can solve problems such as large side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
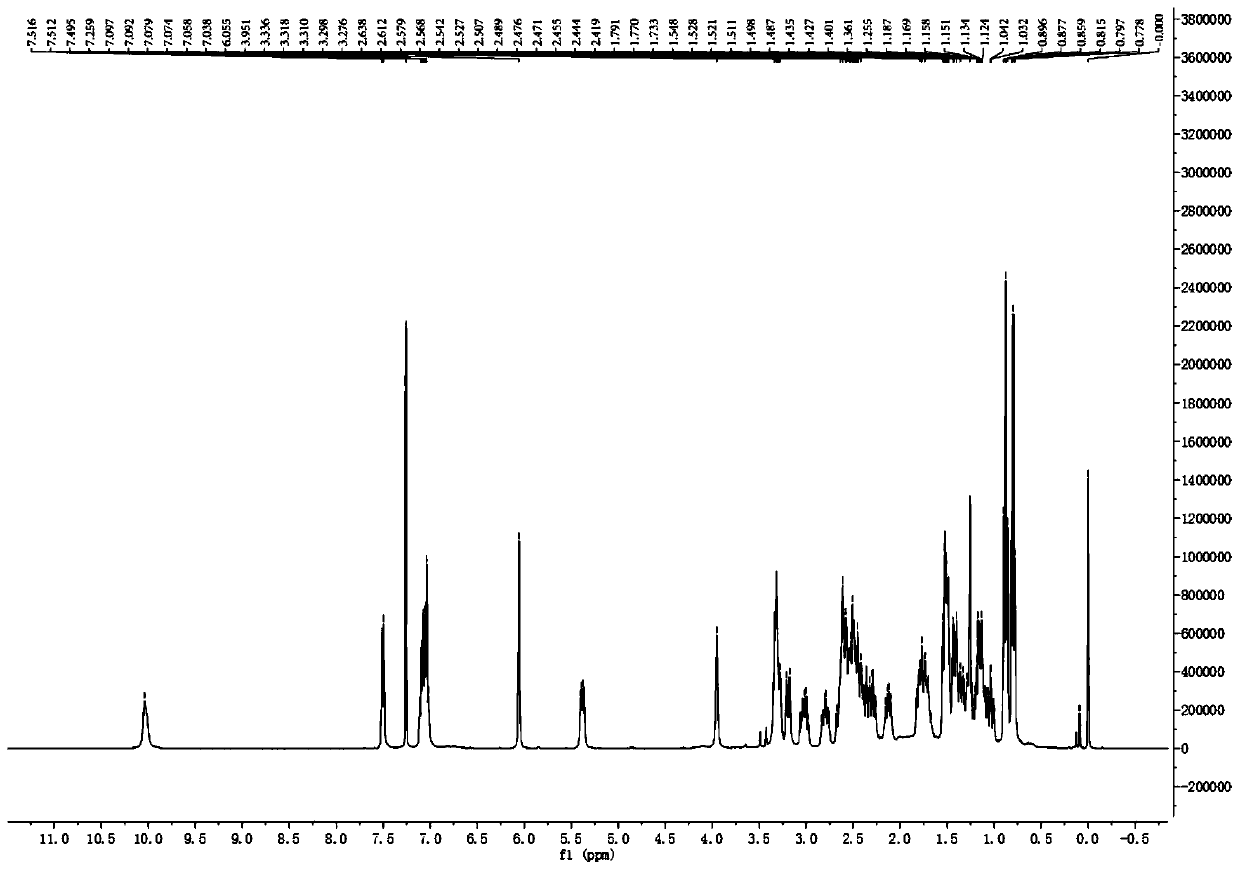
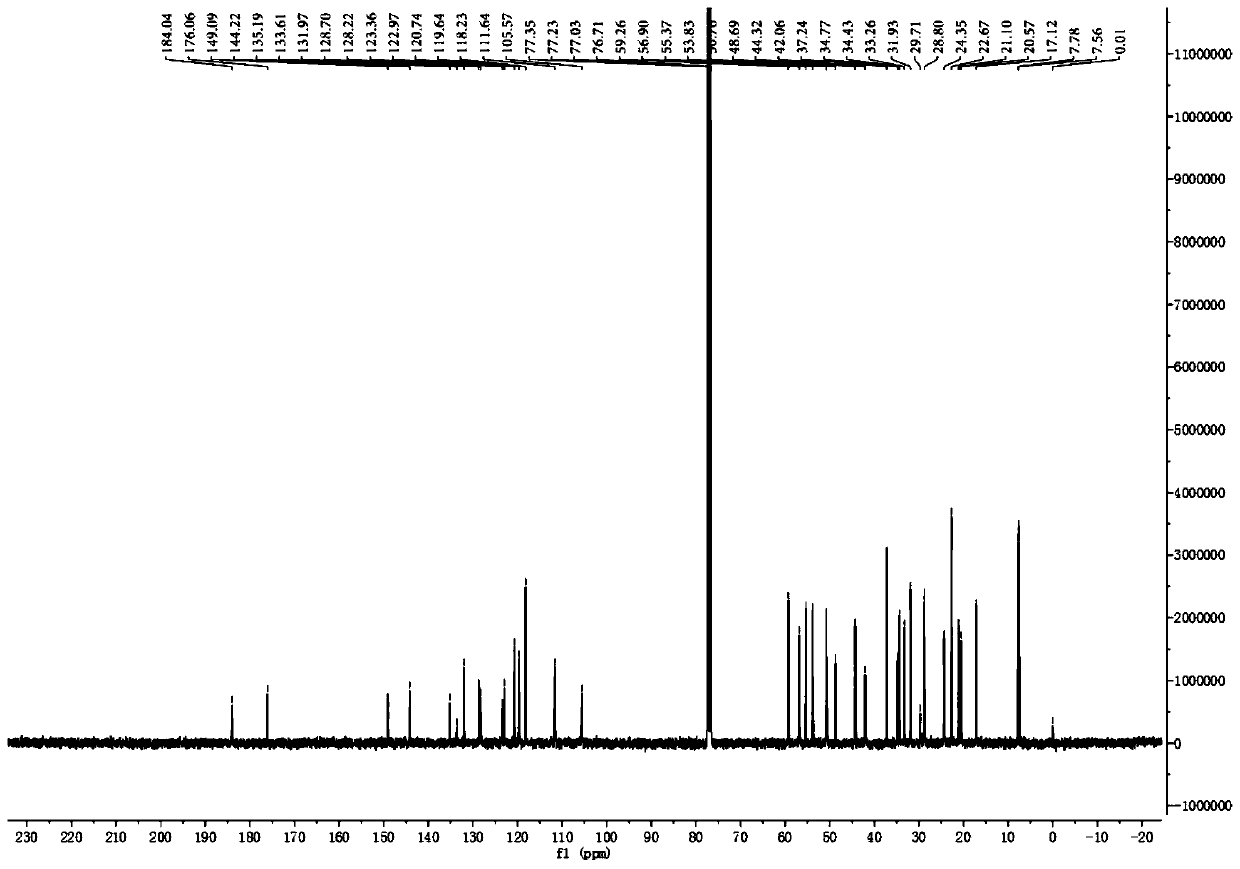
[0094]Take 18kg of air-dried rhizomes of the milk vine plant, heat and reflux with 95% ethanol to extract 4 times, recover the solvent until there is no alcohol smell, add water to suspend, adjust the pH to 2 with 2% HCl, extract 4 times with dichloromethane, The aqueous layer was adjusted to pH 10 with 10% NaOH, extracted 4 times with dichloromethane, recovered dichloromethane to obtain total alkaloids, mixed with silica gel, 200-300 mesh silica gel column chromatography, divided into 8 sections (C1-C8 Section), C1 section uses petroleum ether / acetone 20:1, 15:1, 10:1, 5:1, 2:1, 1:1 as eluent, 150g silica gel column chromatography, through thin layer chromatography (TLC ) after pointing the board, it is determined to be divided into 16 sections (C1-1~C1-16), and the C1-13 section is divided into dichloromethane / methanol 100:1, 80:1, 50:1, 40:1, 20:1, 15:1, 10:1, 8:1, 4:1, 2:1 gradient elution, analyzed by TLC spot plate, combined into 3 segments (C1-13A, C1-13B and C1-13C), C...
Embodiment 2
[0106] According to the method of Example 1, firstly obtain bismuthine A, add 4% hydrochloric acid solution to pH = 4, filter and dry to prepare bismuthine A hydrochloride.
Embodiment 3
[0108] According to the method of Example 1, firstly obtain biscarina, add 4% sulfuric acid ethanol solution to pH = 4, filter, dry, and prepare biscarina sulfate.
PUM
| Property | Measurement | Unit |
|---|---|---|
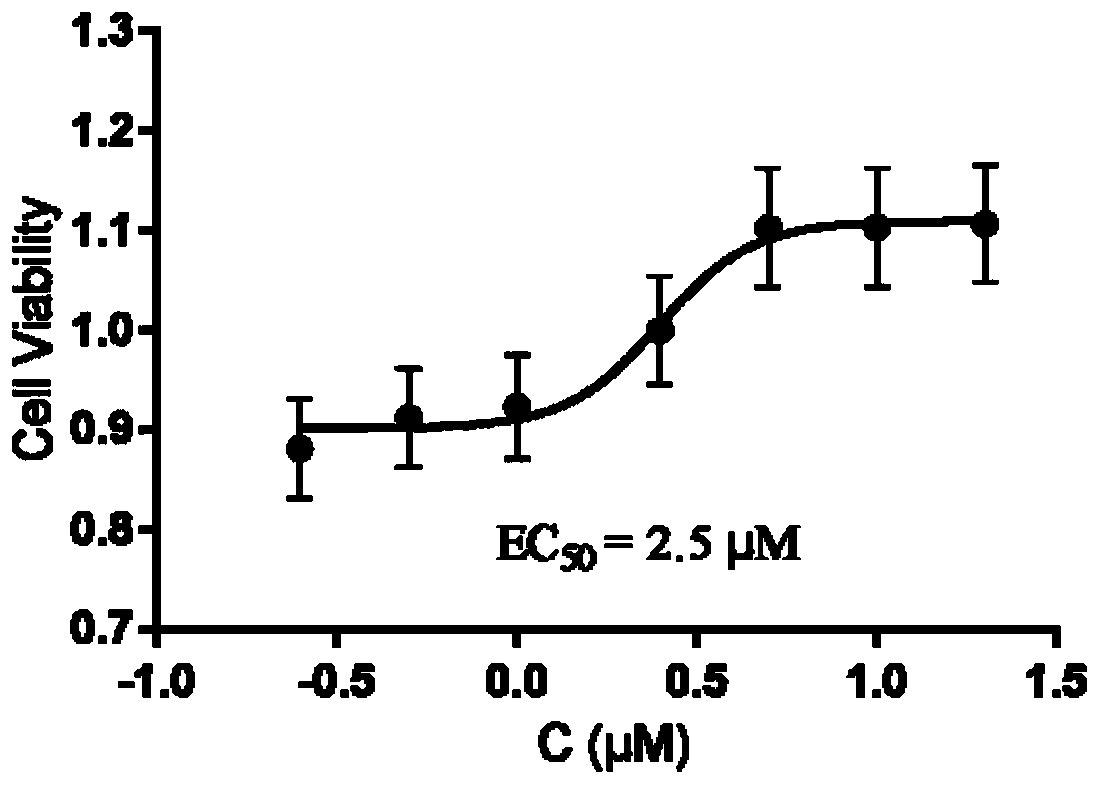
| Half inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com