Phospholipid compound and preparation method thereof
A phospholipid compound, separation and purification technology, applied in the directions of edible phospholipid compositions, chemical instruments and methods, compounds of elements of Group 5/15 of the periodic table, etc. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
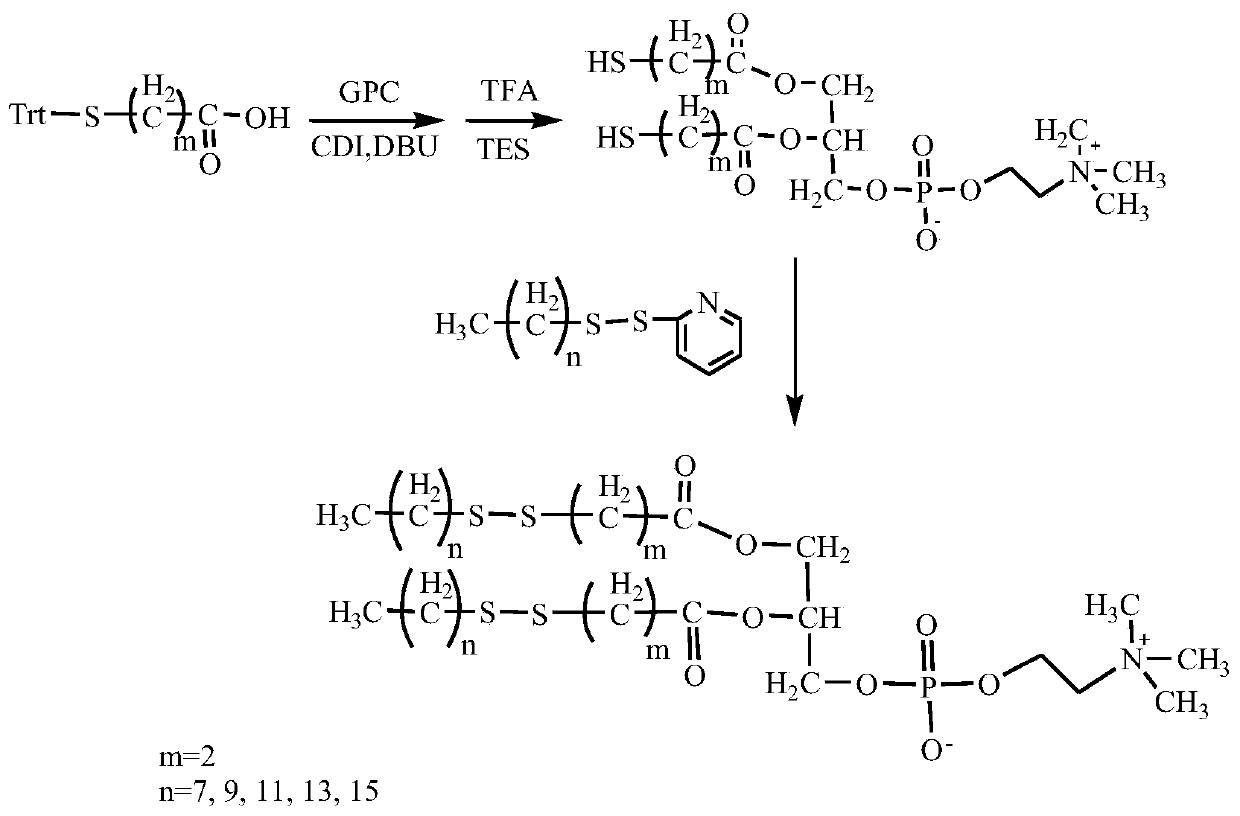
[0037] Its preparation method comprises the following steps (partial bis-n-alkyl dithio-n-alkanoic acid glycerophosphorylcholine synthetic route such as figure 1 shown):
[0038] (1) The trityl-protected mercapto-n-alkanoic acid was dissolved in dimethyl sulfoxide, and a catalytic amount of N,N'-carbonyldiimidazole and 1,8-diazabicyclo[5.4.0]undecyl- 7-ene, add glycerophosphocholine, stir and react at room temperature to 60°C for 1 to 12 hours, after the reaction is completed, add distilled water to the reaction solution, then add dichloromethane for extraction, concentrate the extract and use column chromatography to separate and purify, to obtain The intermediate bis-trityl mercapto-n-alkanoic acid glycerophosphocholine;
[0039] (2) Dissolve bis-trityl mercapto n-alkanoic acid glycerophosphorylcholine in dichloromethane, add appropriate amount of trifluoroacetic acid and triethylsilane, stir and react at room temperature for 0.5 to 6 hours, and remove the protective group ...
Embodiment 1
[0049] Embodiment 1 bis-n-octyl dithiopropionate glycerophosphocholine
[0050] A kind of phospholipid compound, this compound is bis-n-octyl dithiopropionate glycerophosphocholine (in the formula, m gets 2, n gets 7), and its structure is:
[0051]
[0052] Its preparation steps are as follows:
[0053] 1. Dissolve 20 mmol of trityl (Trt)-protected mercaptopropionic acid in 50 ml of dimethyl sulfoxide, add 20 mmol of CDI and 10 mmol of DBU, add 10 mmol of glycerophosphocholine (GPC) , stirred and reacted at room temperature for 6 hours; after the reaction was completed, 50 milliliters of distilled water was added, extracted with 100 milliliters of dichloromethane, the extract was dried over magnesium sulfate, concentrated and purified by silica gel column chromatography to obtain bis-trityl mercaptopropane Glycerylphosphocholine;
[0054] 2. Dissolve 8 mmoles of the above-mentioned bis-trityl mercaptopropionate glycerol phosphorylcholine in 50 ml of dichloromethane, add ...
Embodiment 2
[0058] Embodiment 2 bis-n-decyl dithiopropionate glycerophosphocholine
[0059] A kind of phospholipid compound, this compound is bis-n-decyl dithiopropionate glycerophosphocholine (in the formula, m gets 2, n gets 9), and its structure is:
[0060]
[0061] 1. Dissolve 20 mmol of trityl (Trt)-protected mercaptopropionic acid in 50 ml of dimethyl sulfoxide, add 20 mmol of CDI and 10 mmol of DBU, add 10 mmol of glycerophosphocholine (GPC) , stirred and reacted at room temperature for 12 hours; after the reaction was completed, 50 milliliters of distilled water was added, extracted with 100 milliliters of dichloromethane, the extract was dried over magnesium sulfate, concentrated and purified by silica gel column chromatography to obtain bis-trityl mercaptopropane Glycerylphosphocholine;
[0062] 2. Dissolve 8 mmoles of bis-trityl mercaptopropionin glycerol phosphorylcholine in 50 ml of dichloromethane, add 0.3 ml of trifluoroacetic acid and 0.3 ml of triethylsilane, stir at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com