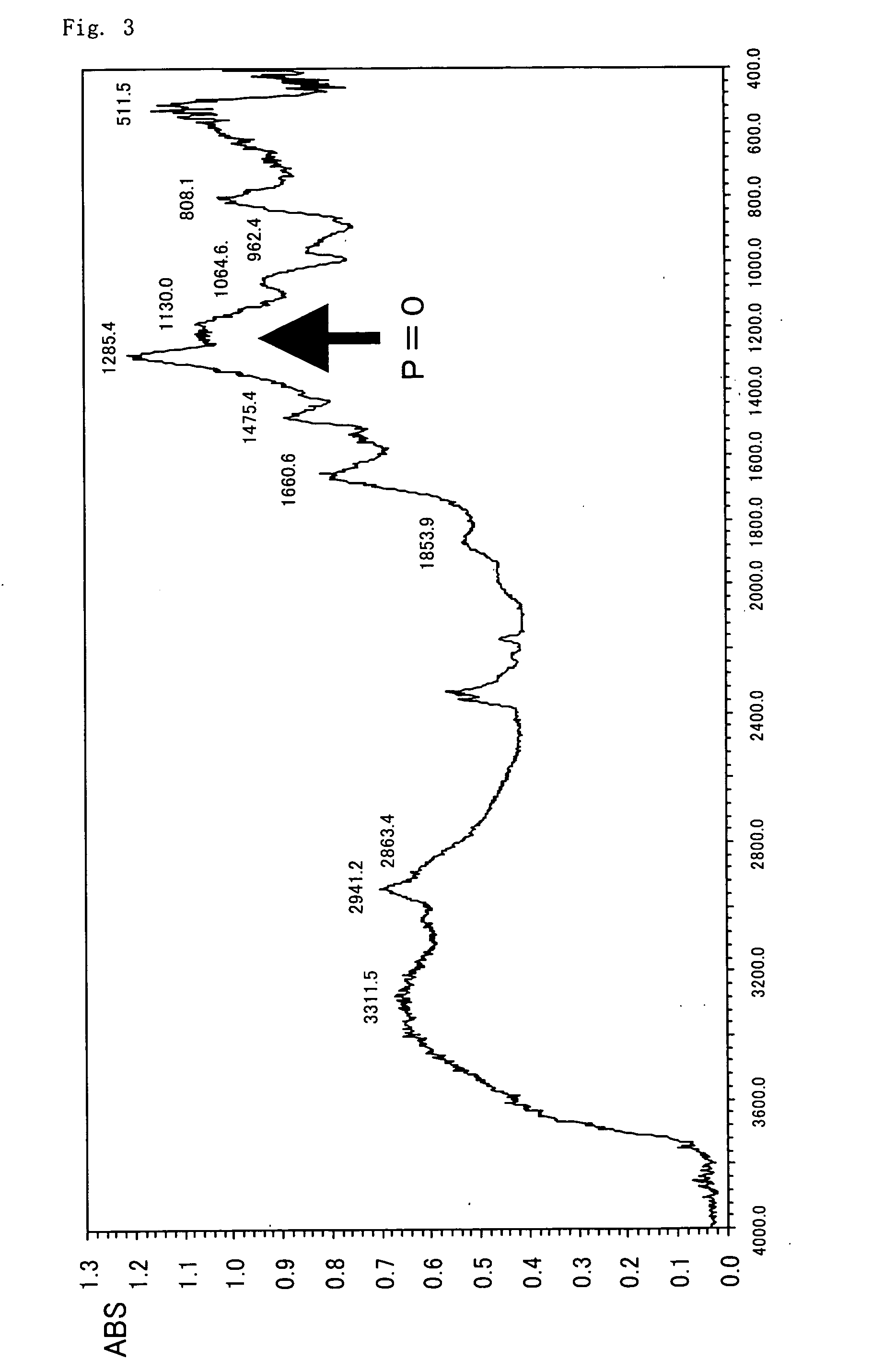
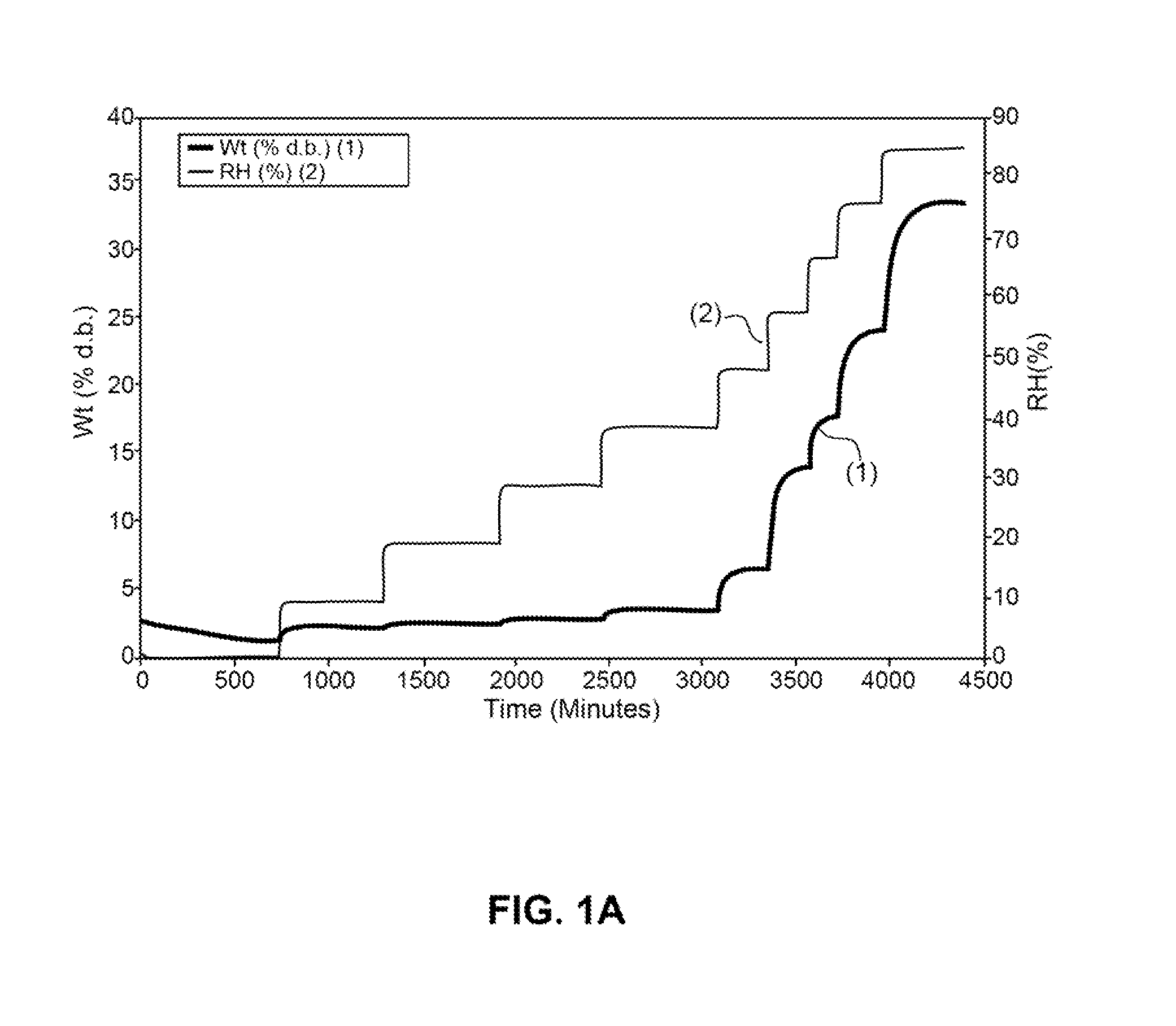
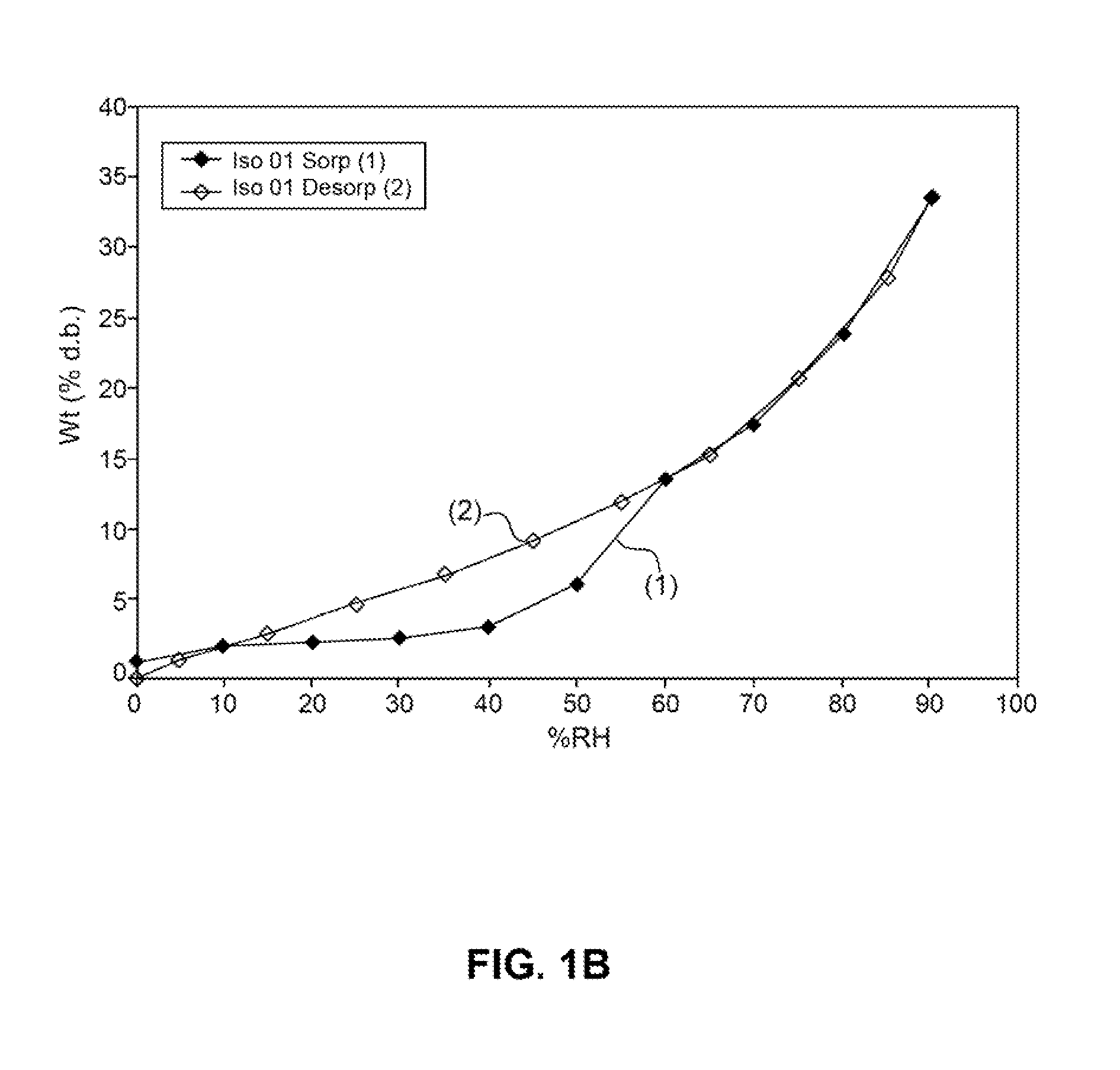
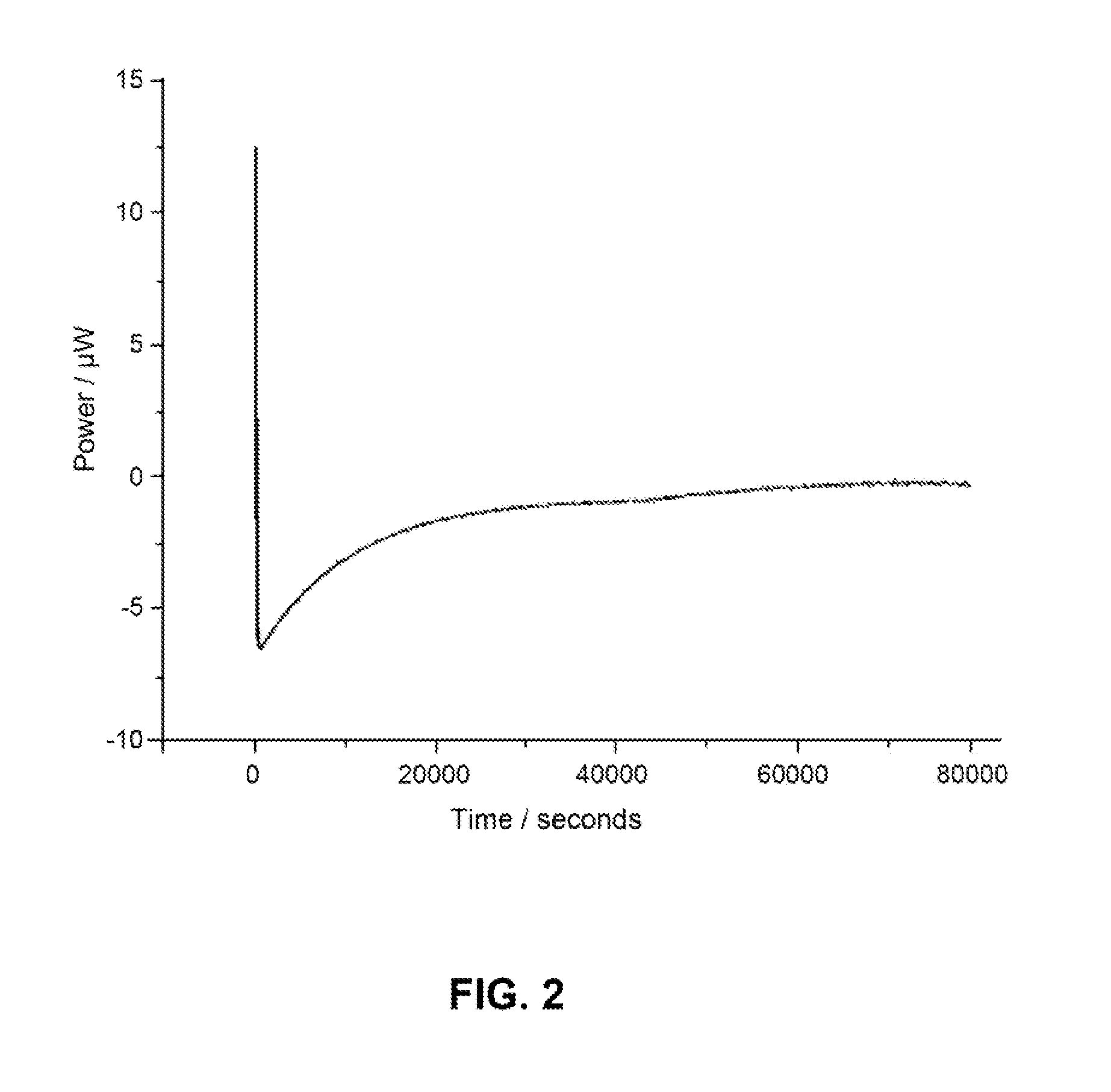
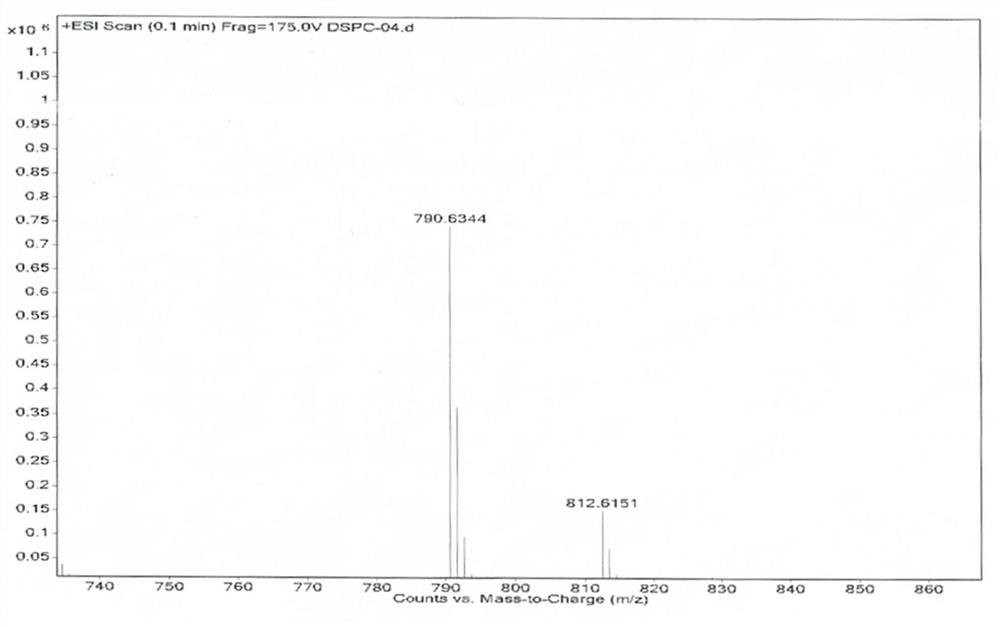
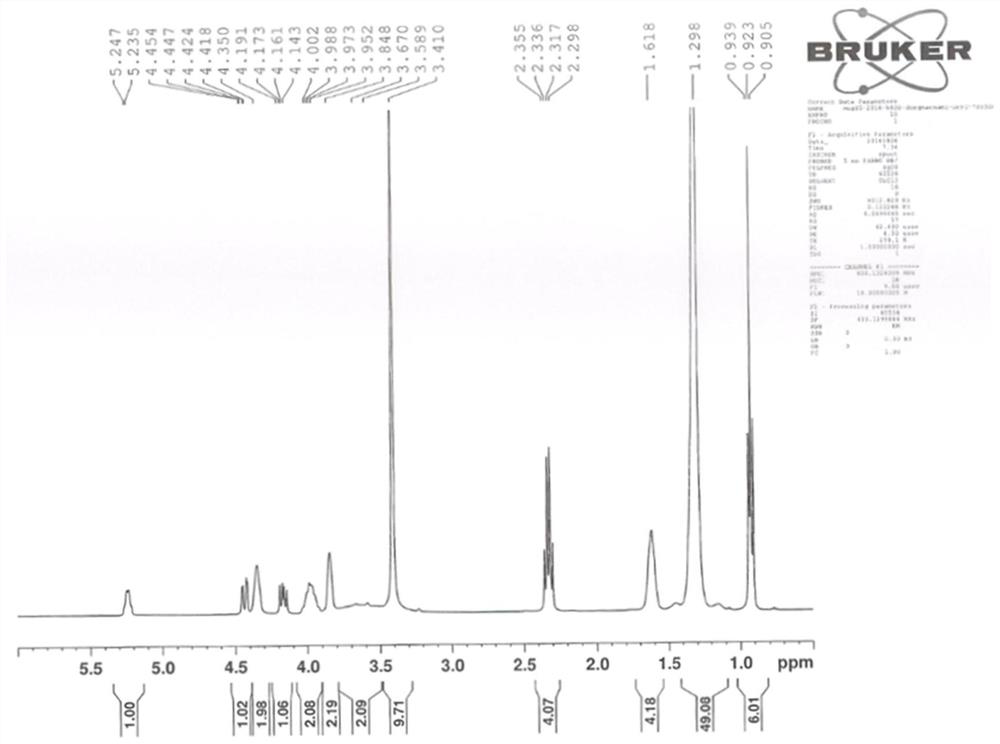
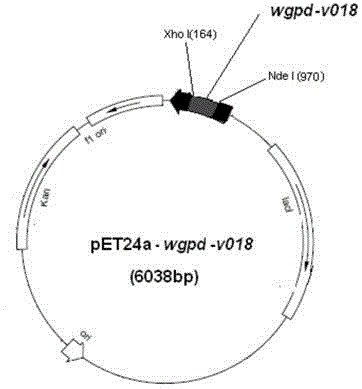
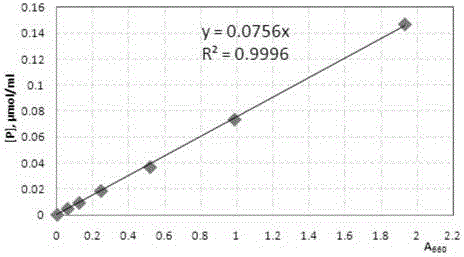
Patents
Literature
51 results about "Choline Glycerophosphate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
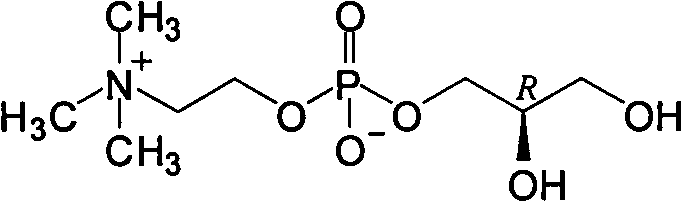
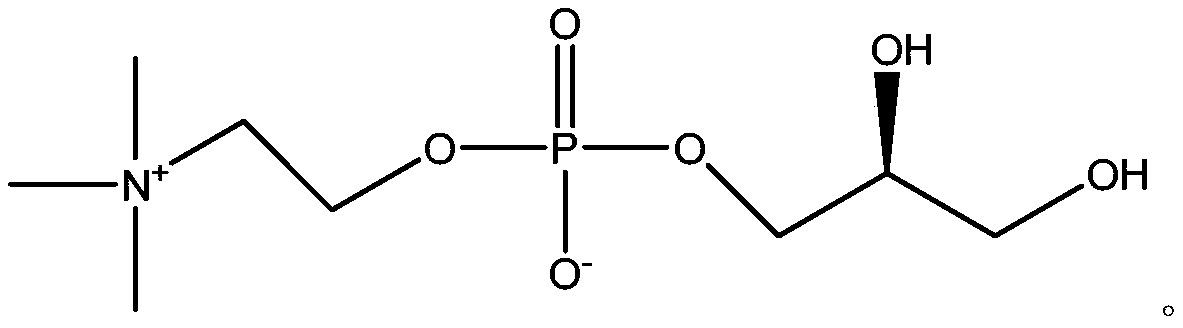
Choline glycerophosphate is a component of PHOSPHATIDYLCHOLINES or LECITHINS, in which the two hydroxy groups of GLYCEROL are esterified with fatty acids.
Gas-filled microbubbles and systems for gas delivery
InactiveUS20140010848A1Eliminate side effectsLow viscosityBiocideBreathing masksDipalmitoylphosphatidylcholinePhosphorylcholine
Compressible and concentrated suspensions containing gas-filled microbubbles, uses thereof for delivering gas into a subject in need thereof, and systems for delivering the compressible suspensions. The gas-filled microbubbles each comprise a gas core surrounded by a lipid membrane, which includes (a) one or more lipids, such as 1,2-disteroyl-sn-glycero-3-phosphocholine (DSPC) or dipalmitoylphosphatidylcholine (DPPC), and (b) one or more stabilizing detergents, such as poloxamer 188, Pluronic F108, Pluronic F127, polyoxyethylene (100) stearyl ether, cholesterol, gelatin, polyvinylpyrrolidone (PVP), and sodium deoxycholate (NaDoc).
Owner:CHILDRENS MEDICAL CENT CORP
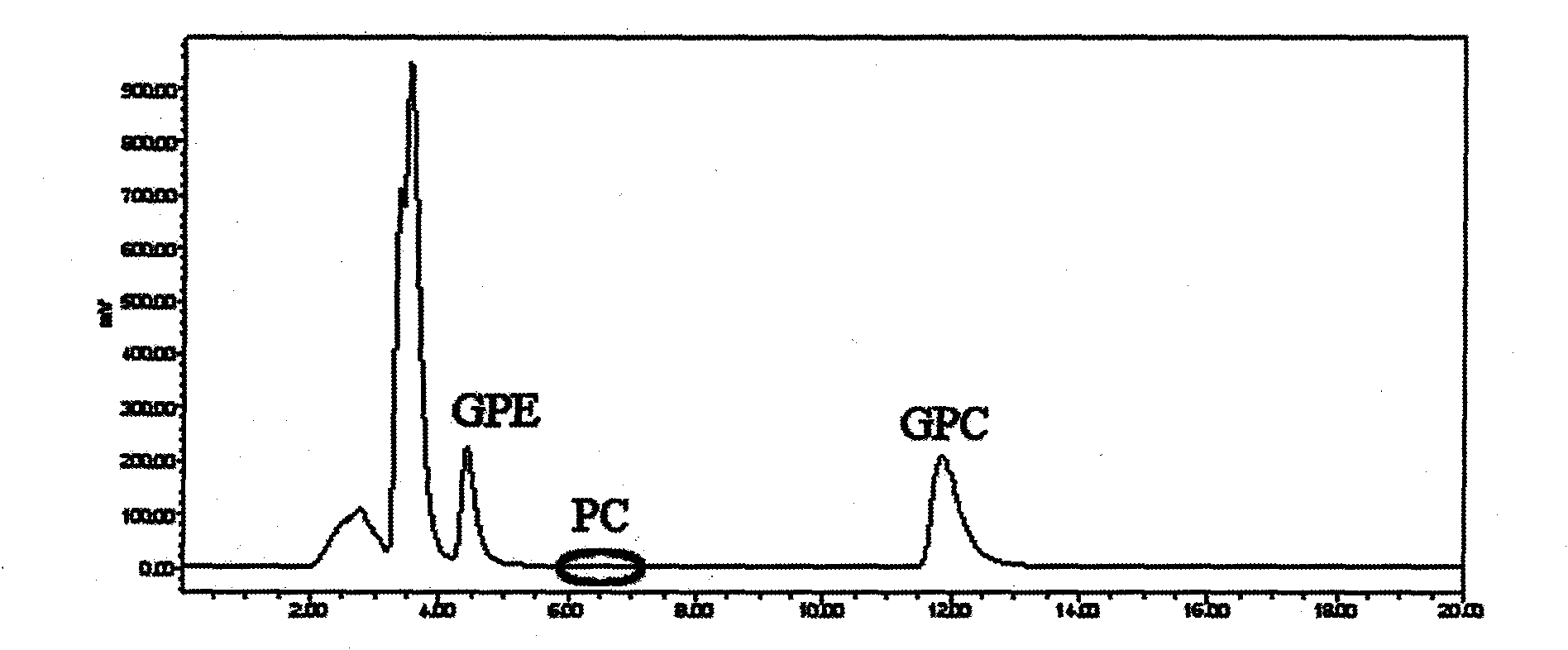
Method for separating and purifying L-alpha-glycerophosphorylcholine (L-alpha-GPC) by silica gel column chromatography
ActiveCN102093410ALow costGuaranteed optical activityFermentationPhosphorus organic compoundsChromatographic separationPurification methods
The invention discloses a method for separating and purifying L-alpha-GPC by silica gel column chromatography, which belongs to the technical field of lipid development and application and comprises the following steps: removing Ca<2+> and Cl<-> from enzymatic hydrolysis reaction solution serving as a raw material by using ion exchange resin, converting a water phase into an alcohol phase, separating L-alpha-GPC from glycerol polyglycidyl ether (GPE), lysophosphatidylcholine (LPC) and other byproducts, decolorizing by active carbon, and dewatering through vacuum concentration to obtain a colorless and transparent product; directly passing alcoholysis reaction solution serving as a raw material through a silica gel column for separation, removing Na<+> by cation exchange resin, decolorizing by active carbon, and dewatering through vacuum concentration to obtain a product. According to the test of the indexes of the product, the chemical purity is 99.6 percent, the optical purity ee is 99 percent, and the melting point (mp) is 142 to 143 DEG C (wherein C is equal to 2.6, the H2O content is 16 percent, and the pH value is 5.8). The invention provides a new way of thought and a new method for separating and purifying L-alpha-GPC, and realizes the application of the silica gel column chromatographic separation and purification method in lipid science.
Owner:JIANGNAN UNIV +1
Method of modifying surface of material
InactiveUS20060060533A1Easy to adjustIncrease chanceIon-exchange process apparatusOther chemical processesPhosphorylcholinePhosphoric acid
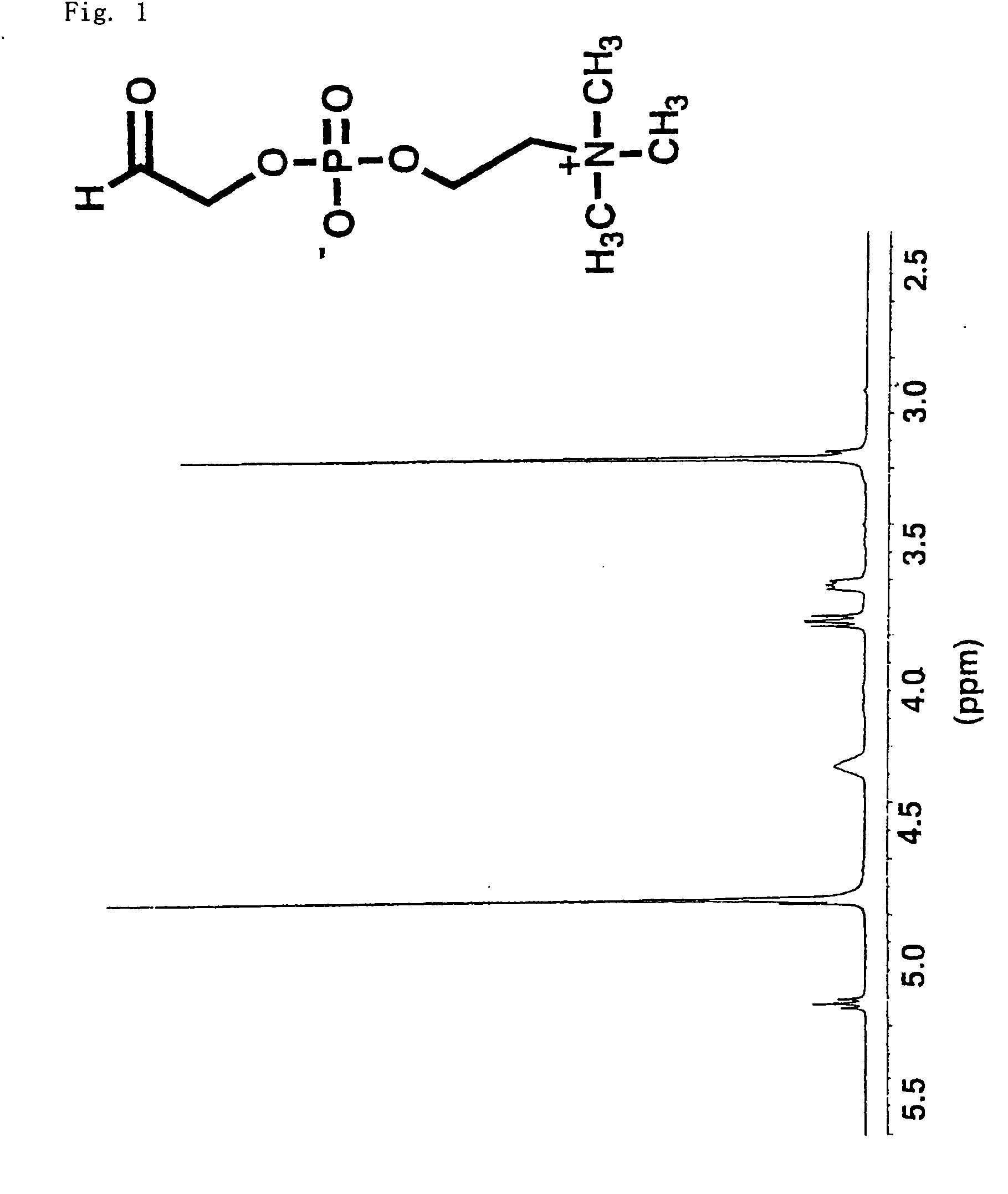
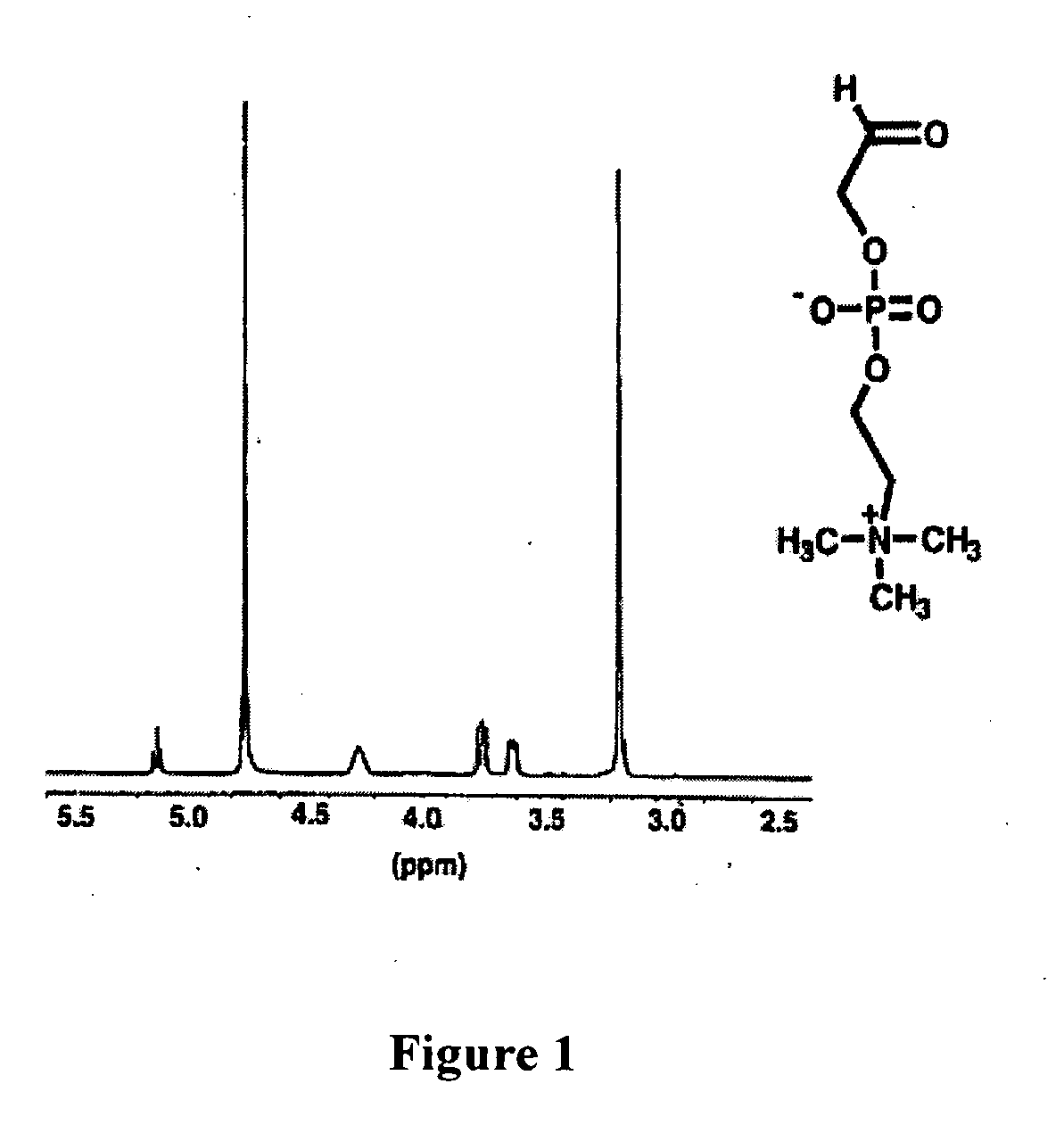
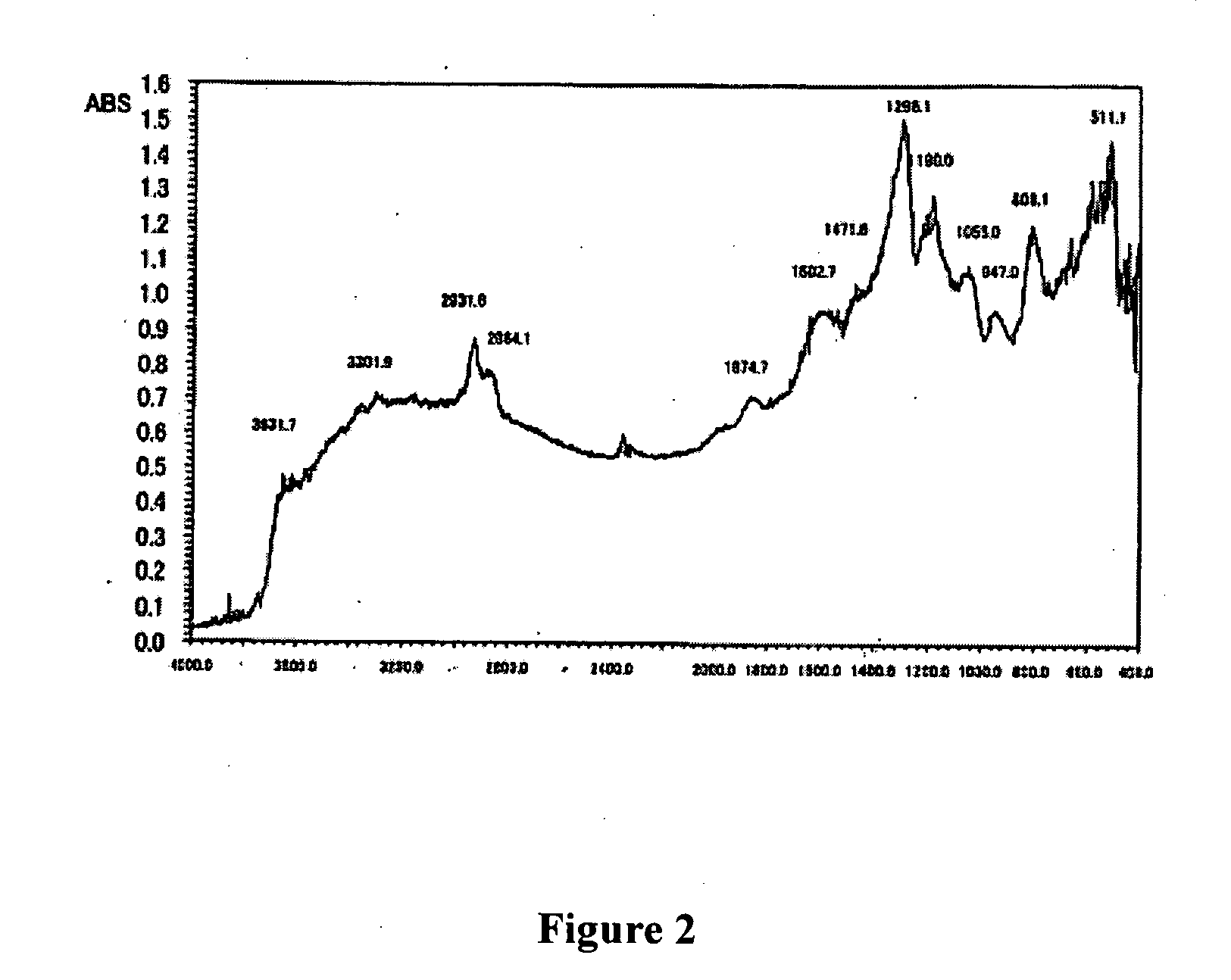
A method for surface modification of a material by means of introducing the phosphorylcholine group represented by the following formula (1-1) onto the surface of the material by treating a material having amino groups with a chemical compound containing an aldehyde derivative obtained by the oxidative ring-opening reaction of glycerophosphorylcholine. The method of the present invention provides various materials such as medical materials having superior biocompatibility and hydrophilicity.
Owner:SANYO FINE IRICA TECH CO LTD
Formulations and Dosage Forms of Oxidized Phospholipids
ActiveUS20130209555A1Prevent leakage and crackingPreserve homogeneityOrganic active ingredientsNervous disorderDrugOxidized phospholipid
The current disclosure provides pharmaceutical compositions containing an oxidized phospholipid, such as 1-hexadecyl-2-(4′-carboxybutyl)-glycero-3-phosphocholine (VB-201) and a thermosoftening carrier, e.g., a poloxamer. The pharmaceutical compositions may further comprise an anti-adherent agent, such as talc and / or a thixotropic agent. The current disclosure further provides processes for preparing the pharmaceutical compositions. The disclosure further provides capsules containing the pharmaceutical compositions. Uses of such pharmaceutical compositions and capsules in treating inflammatory disorders are also disclosed.
Owner:VASCULAR BIOGENICS
Method for preparing glycerophosphorylcholine (GPC) by phospholipase-catalyzed hydrolysis
The invention discloses a method for preparing GPC by phospholipase-catalyzed hydrolysis and belongs to the technical field of lipid development and application. The method comprises the following steps: preparing GPC by using powdered phospholipid, alcohol soluble phospholipids, high-purity PC as raw materials and by phospholipase-catalyzed hydrolysis of phosphorylcholine (PC) in a water phase; and decolorizing by using active carbon to obtain GPC aqueous solution, converting into an alcohol phase, purifying by using a cation resin adsorption resolution and anion adsorption process to obtain high-purity GPC aqueous solution, performing rotating evaporation at a low temperature and under reduced pressure to obtain colorless transparent L-alpha-GPC solution, wherein the chemical purity of the obtained GPC is 98.8 percent, the optical purity ee of the obtained GPC is 99 percent, and the melting point (mp) of the obtained GPC is 142 to 143 DEG C (C is equal to 2.6, the H2O content is 14 percent and the pH value is 5.8). The invention provides a new thought and a new preparation method for preparing GPC and provides new application of phospholipase in lipid science.
Owner:JIANGNAN UNIV
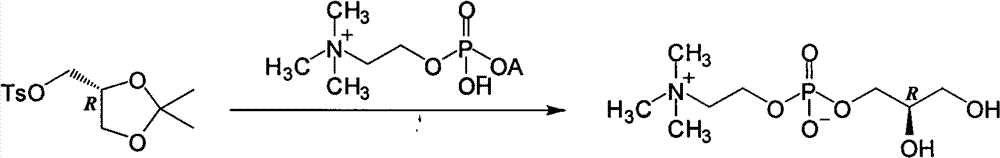
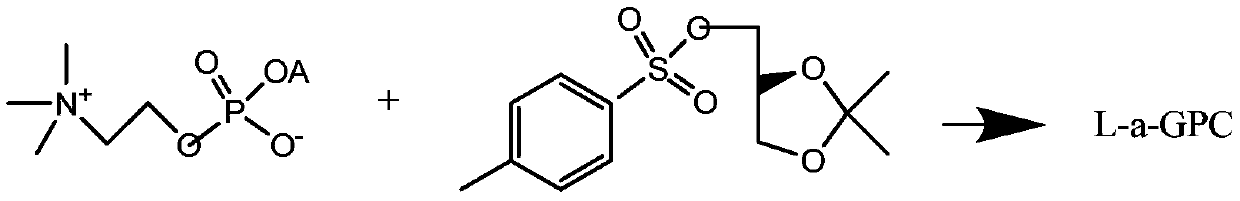
Preparation method of L-alpha-choline glycerophosphate
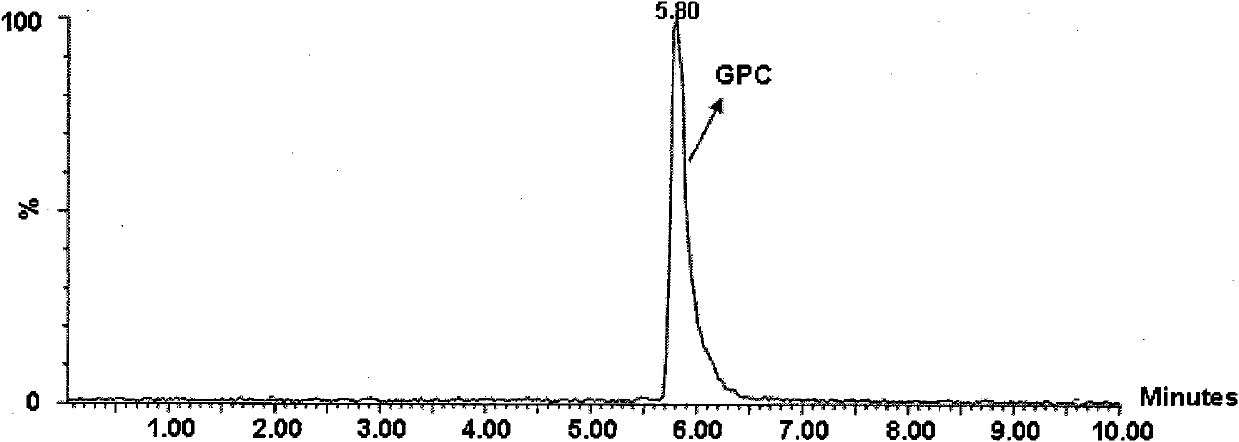
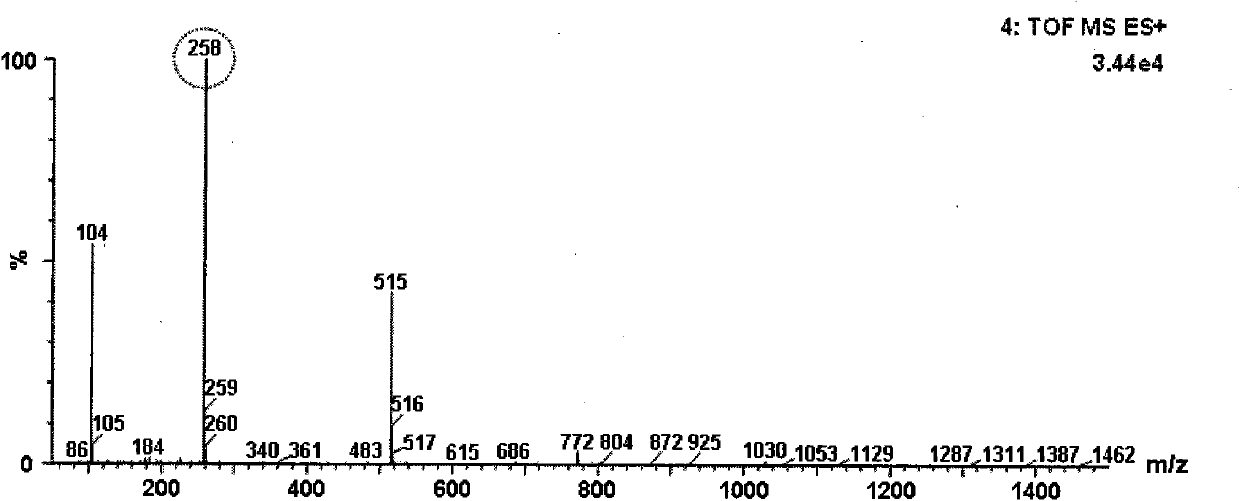
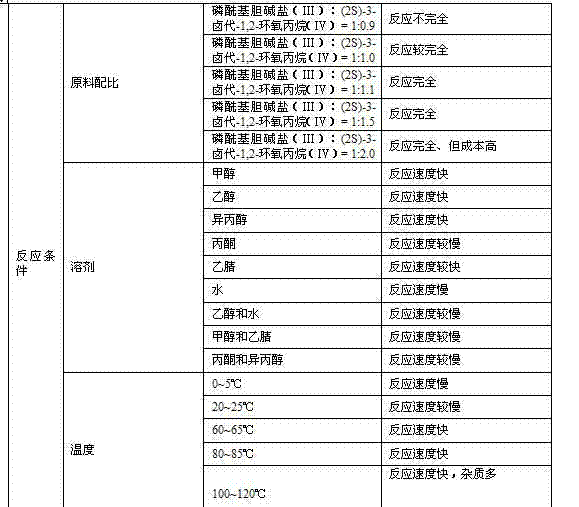
ActiveCN103665028AQuality improvementSimple and fast operationPhosphorus organic compoundsPhosphoryl cholineIon exchange
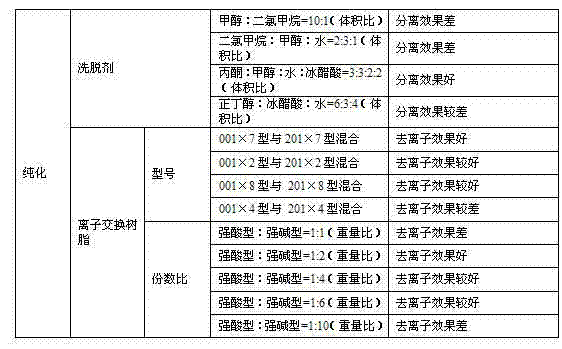
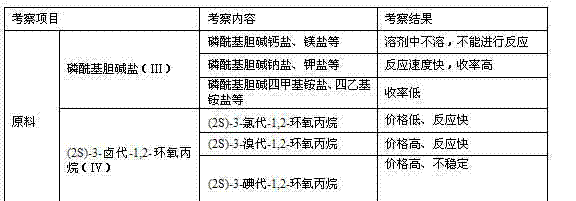
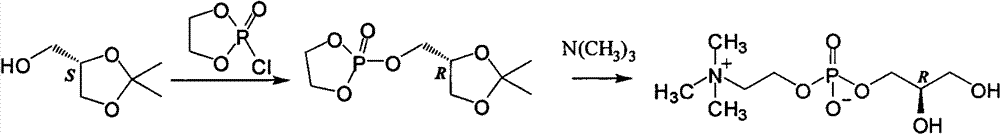
The invention discloses a preparation method of L-alpha-choline glycerophosphate. According to the method, (2S)-3-halogenated-1,2-propylene oxide and phosphoryl choline salt are used as the raw materials, and are subjected to esterification and hydrolysis reaction in a solvent to obtain the L-alpha-choline glycerophosphate crude product, and the crude product is subjected to silica gelcolumn chromatography and purification through ion exchange resin to obtain the L-alpha-choline glycerophosphate pure product. Compared with the prior art, the preparation method disclosed by the invention has the advantages that the operation is simpler; the adopted chiral material (2S)-3-halogenated-1,2-propylene oxide is cheap and easy to obtain; the prepared crude product is subjected to silica gelcolumn chromatography and purification through ion exchange resin to obtain the L-alpha-choline glycerophosphate product with higher quality, and the L-alpha-choline glycerophosphate content is more than 99%; the technical process is more suitable for large scale production.
Owner:天津市医药集团技术发展有限公司
A method for preparing lysophosphatidylcholine by enzymatic alcoholysis
InactiveCN102277393AImprove conversion rateImprove solubilityFermentationPhosphoric Acid EstersPhosphoric acid
The invention discloses a method for preparing lysophosphatidyl choline by enzymatic alcoholysis, which comprises: adding phosphatidylcholine into low-carbon alcohol solution, uniformly stirring and mixing at a certain temperature, adding lipase, and allowing the lipase to catalyze the alcoholysis of phosphatidylcholine with constant-temperature stirring to obtain the lysophosphatidyl choline. The method has the advantages that: (1) the solubility of phosphatidylcholine, lysophosphatidyl choline serving as the product, and aliphatic ester, glycerophosphorylcholine and very small amount of fatty acid, which serve as byproducts, in the reaction system is improved, and the conversion rate of the lysophosphatidyl choline is high; (2) the aliphatic ester, a small amount of glycerophosphate and a very small amount of fatty acid are generated in an alcoholysis reaction process, but the pH value of the system is not changed, and the influence of the solvent on the activity of the lipase is relieved and the catalytic reaction activity of the lipase is high; (3) the properties of the fatty acid, the aliphatic ester and glycerophosphorylcholine, which are products of side reactions, are very different from those of the lysophosphatidyl choline, so the lysophosphatidyl choline product can be separated and purified very easily; and (4) the alcohol, which is the product of the reaction, can be removed easily.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
L-alpha-choline glycerophosphate synthesis method
InactiveCN103087091AOvercome stabilityPhosphatide foodstuff compositionsSynthesis methodsCholine Phosphate
The present invention relates to a method, which comprises that (R)-(-)-3-chloro-1,2-propanediol and a phosphocholine tetramethyl ammonium salt are subjected to a substitution reaction, and ion exchange resin purification is performed to obtain a L-alpha-choline glycerophosphate pure product. According to the present invention, the used chiral intermediate (R)-(-)-3-chloro-1,2-propanediol is further a key chiral intermediate of an antitussive agent levodropropizine, has characteristics of stable chemical property and convenient and easy obtaining, and is especially for L-alpha-choline glycerophosphate industrial production.
Owner:SHANGHAI CHENPON PHARM TECH CO LTD
Formulations and dosage forms of oxidized phospholipids
ActiveUS9254297B2Maintain homogeneityPrevent leakageOrganic active ingredientsNervous disorderMedicineOxidized phospholipid
The current disclosure provides pharmaceutical compositions containing an oxidized phospholipid, such as 1-hexadecyl-2-(4′-carboxybutyl)-glycero-3-phosphocholine (VB-201) and a thermosoftening carrier, e.g., a poloxamer. The pharmaceutical compositions may further comprise an anti-adherent agent, such as talc and / or a thixotropic agent. The current disclosure further provides processes for preparing the pharmaceutical compositions. The disclosure further provides capsules containing the pharmaceutical compositions. Uses of such pharmaceutical compositions and capsules in treating inflammatory disorders are also disclosed.
Owner:VASCULAR BIOGENICS
Process for preparing choline glycerophosphatide (GPC) with non-aqueous phase enzymatic method
The invention discloses a process for preparing choline glycerophosphatide (GPC) with a non-aqueous phase enzymatic method. The process comprises the steps that: lecithin is dissolved in a heptane solvent; an enzyme-containing solution containing phospholipase A1 and an acetic acid / acetate buffer solution is dropped into the solution; the mixture is subjected to a constant-temperature reaction under a temperature of 30-60 DEG C; a reaction liquid is extracted; and an aqueous phase is concentrated and is dried by baking, so that the GPC product is obtained. In the adopted lecithin, the content of phosphatidyl choline is no lower than 50%. An adopted extractant is a mixed solvent of CH3OH and water. The invention provides the process for preparing choline glycerophosphatide (GPC) with the non-aqueous phase enzymatic method. The process adopts an appropriate reaction system, and assists in preparing GPC products with high yield and high purity. Therefore, domestic existing GPC production processes are fundamentally changed, and GPC products with excellent quality can be prepared highly efficiently with mild conditions.
Owner:曹明成 +1
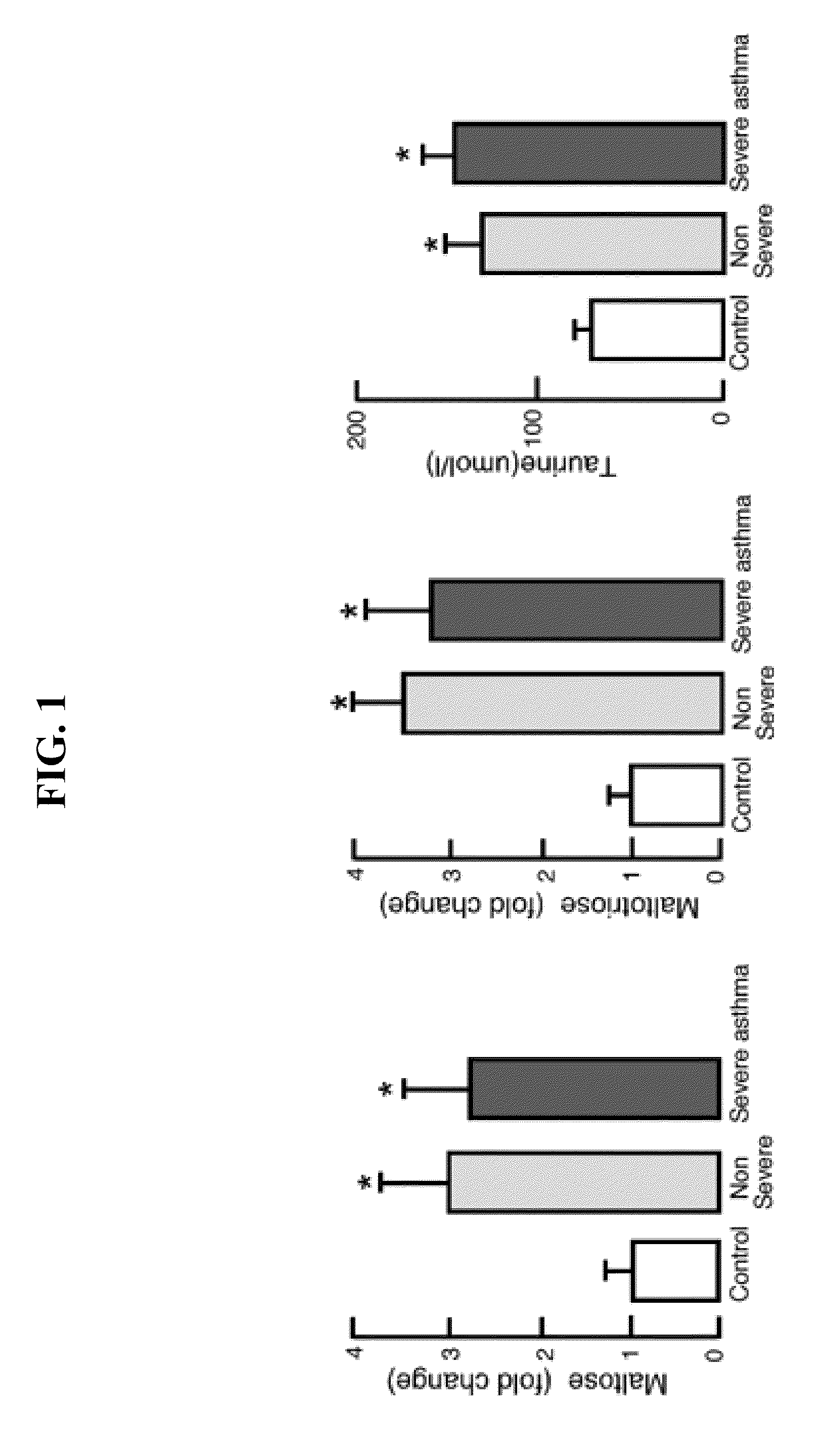
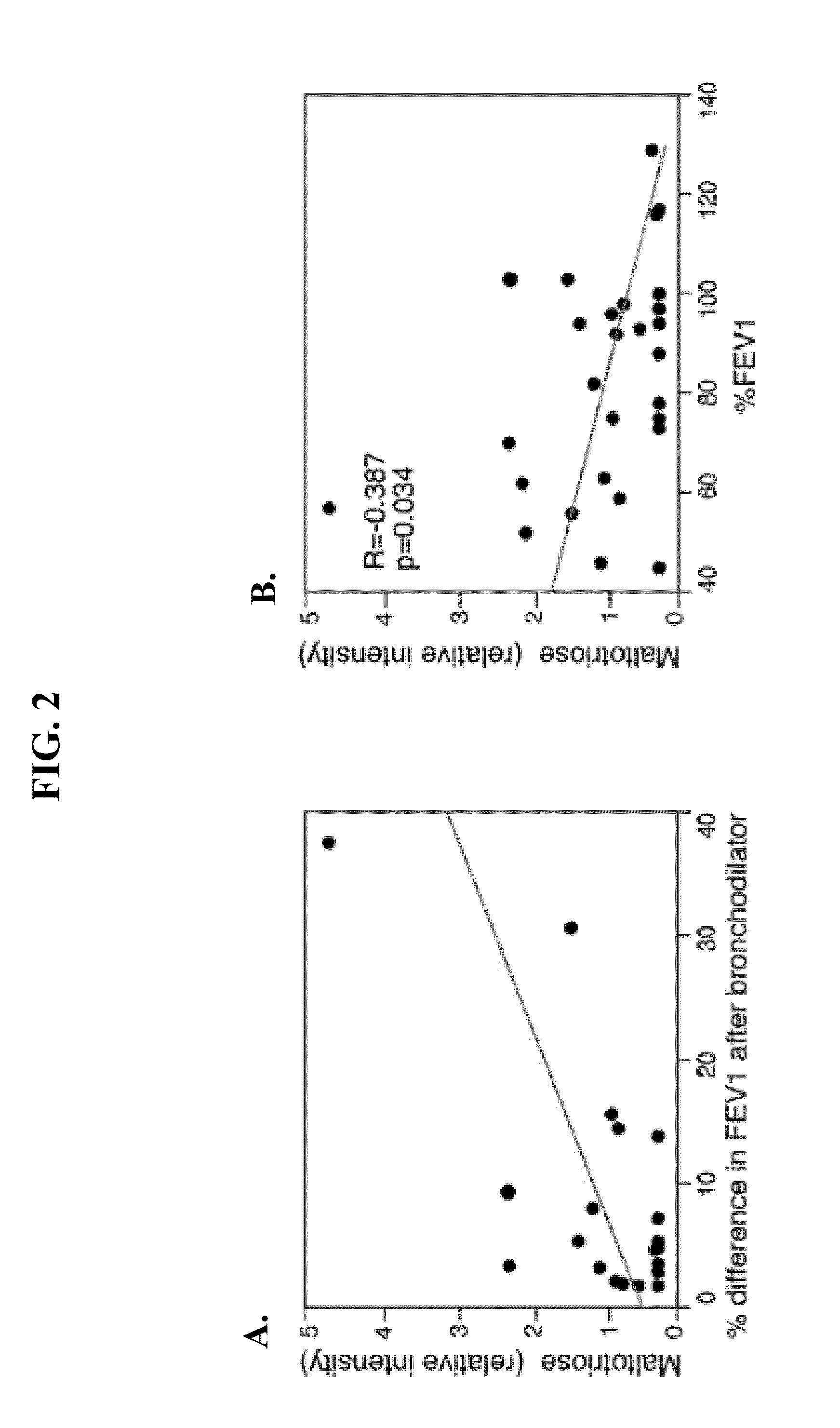
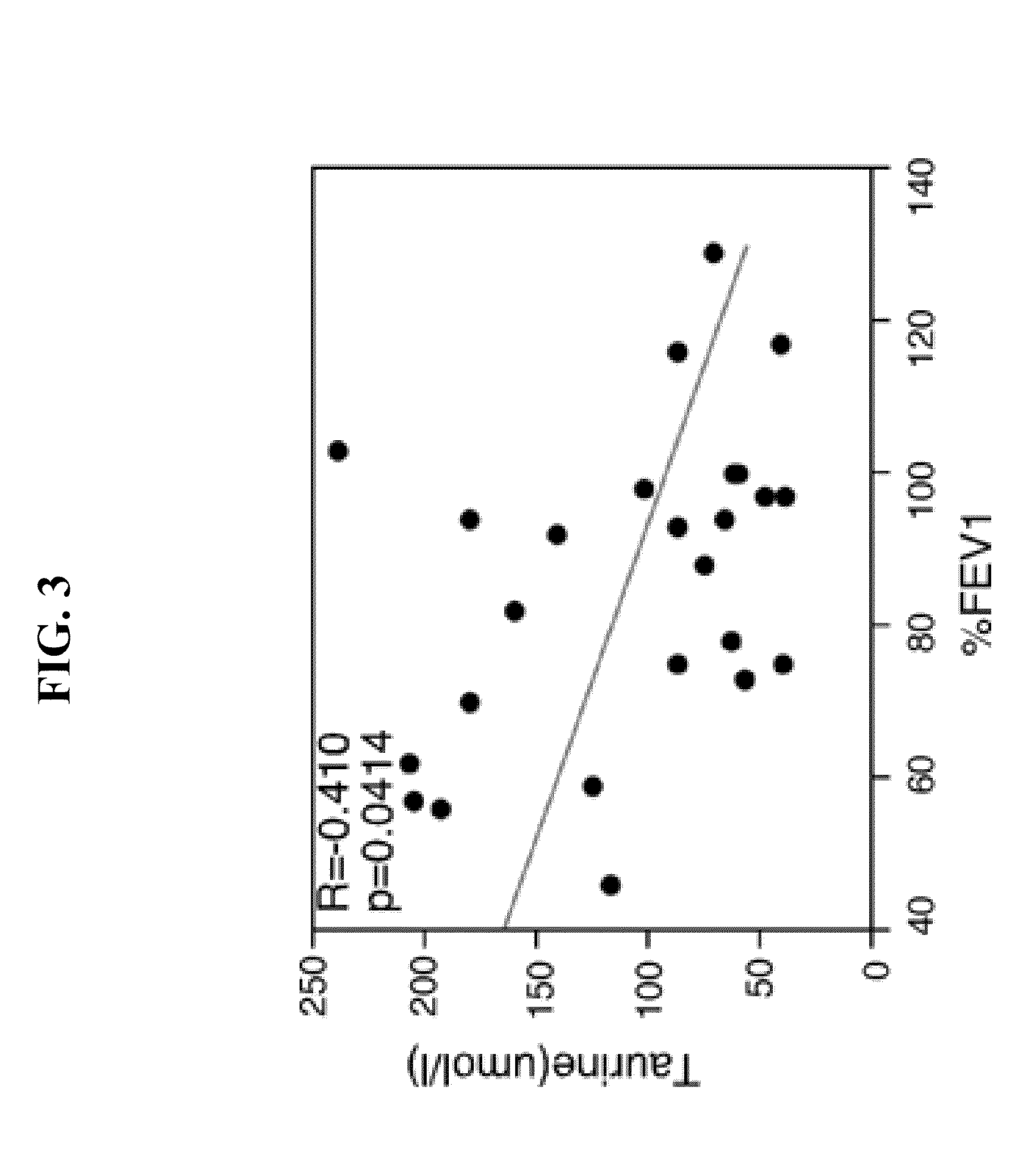
Biomarkers for asthma
The present invention provides methods, kits, and compositions related to testing a sample for the level of a biomarker related to asthma, wherein the biomarker is selected from: taurine, maltose, maltotriose, adenosine 5′-monophosphate, phosphoethanolamine, glycerophosphorylcholine, arachidonate, heptanoate, pelargonate, and nicotinamide. In certain embodiments, the level of the biomarker is used to identify therapy effective for treating asthma. In other embodiments, the level of the biomarker is used to identify the presence, severity, or risk of exacerbation of asthma. In further embodiments, the level of the biomarker is used to monitor the response to on-going therapy (e.g., adjust the dosage of the asthma therapy).
Owner:THE CLEVELAND CLINIC FOUND
Preparation method of choline glycerophosphate
InactiveCN107298692AHigh purityEasy to operatePhosphorus organic compoundsTetrabutylammonium hydroxideSoybean Phospholipids
The invention discloses a preparation method of choline glycerophosphate. The preparation method comprises the following steps: 1) mixing and filtering soybean phospholipid and methyl alcohol, removing a filter residue to obtain a clear liquid M1; 2) adding a tetrabutylammonium hydroxide solution into the clear liquid M1 prepared in the step 1), and stirring and mixing for 30-100 minutes under the condition that the stirring speed is 30-100 r / min, thereby obtaining a mixture M2; 3) adding acetone into the mixture M2 prepared in the step 2), mixing, and removing an acetone layer, thereby obtaining a crude extract M3; 4) separating the crude extract M3 prepared in the step 3) through thin layer chromatography, and collecting, thereby obtaining the choline glycerophosphate. Through the design, the extraction method is simple in operation, the purity of the prepared choline glycerophosphate is relatively high, and the production efficiency is greatly improved.
Owner:WUHU FOMAN BIOPHARMA CO LTD
Phospholipid compound and preparation method thereof
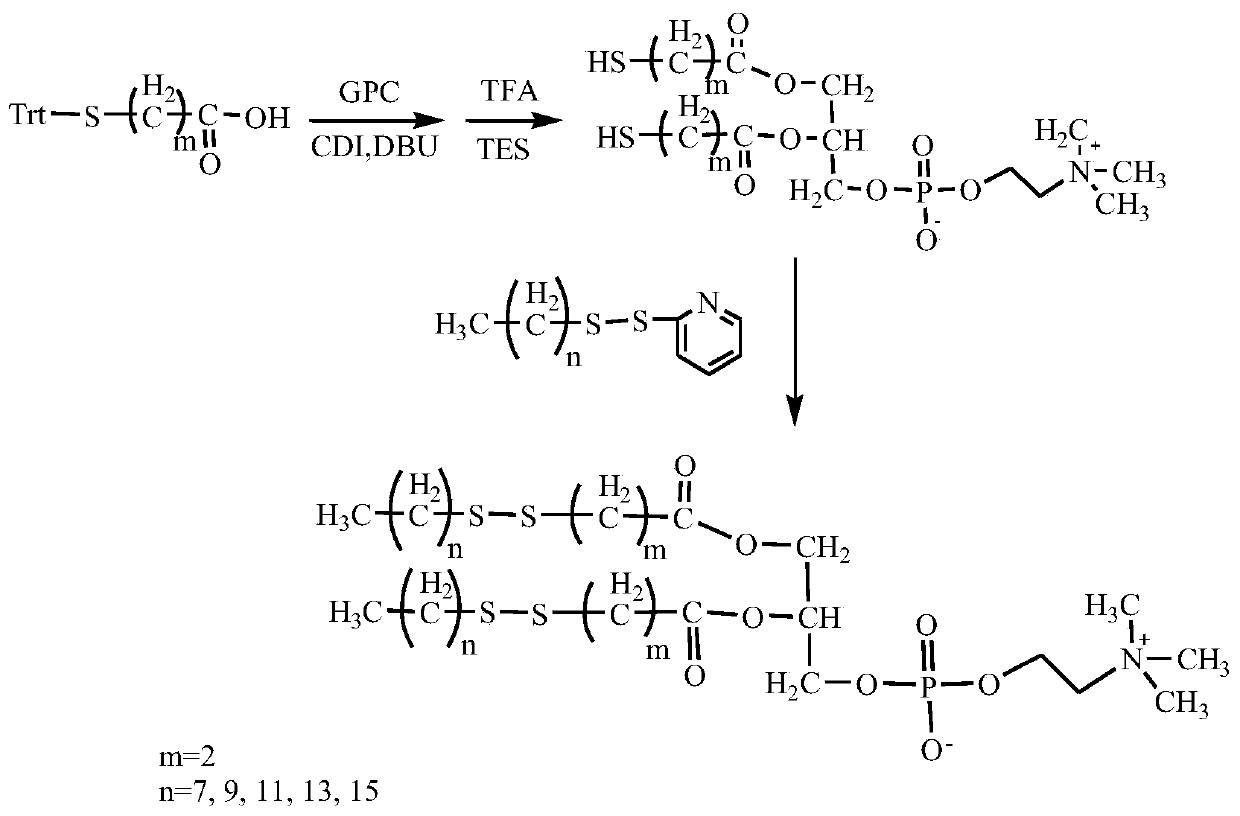
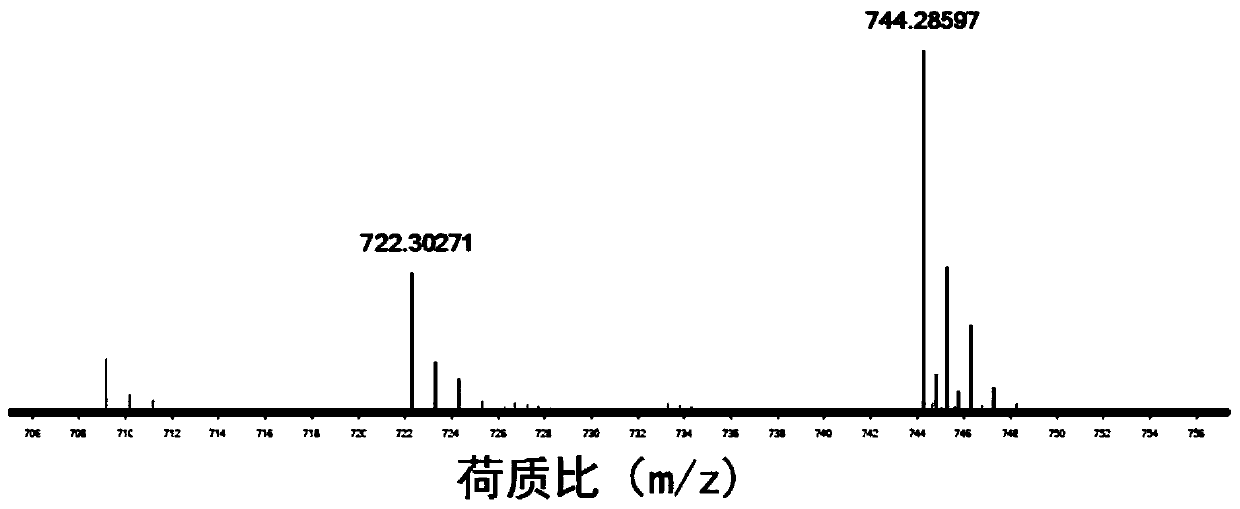
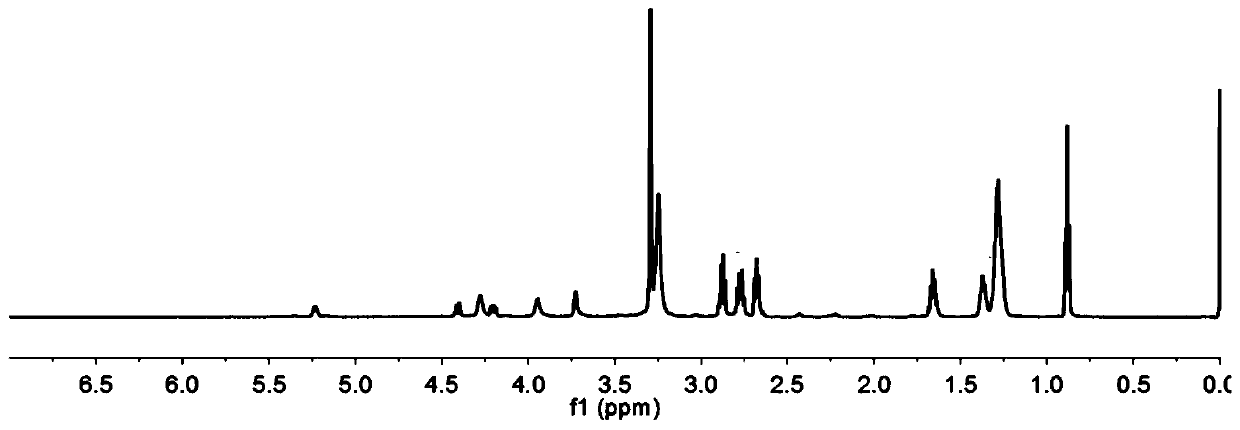
InactiveCN110256485ARapid Fracture DegradationGroup 5/15 element organic compoundsPhosphatide foodstuff compositionsCarbonyldiimidazoleDisulfide bond
The invention discloses a phospholipid compound and a preparation method thereof. The structural general formula of the phospholipid compound is shown in the specification. In the formula, m is a positive integer from 2 to 10, and n is a positive integer from 7 to 15. The compound contains a disulfide bond easy to break and can be quickly broken and degraded in a reducing medium. The method comprises the following steps: (1) dissolving triphenylmethyl protected mercapto n-alkanoic acid into dimethyl sulfoxide, adding N,N'-carbonyl diimidazole, 1,8-diazabicyclo[5.4.0]undecane-7-ene and choline glycerophosphate, and carrying out a reaction to obtain bis-triphenylmethyl mercapto n-alkanoic acid choline glycerophosphate; 2) dissolving the bis-triphenylmethyl mercapto n-alkanoic acid choline glycerophosphate in dichloromethane, adding trifluoroacetic acid and triethylsilane, and carrying out a reaction to obtain bis-mercapto-alkanoic acid choline glycerophosphate; and 3) dissolving the bis-mercapto-alkanoic acid choline glycerophosphate in dichloromethane, adding n-alkyl dithiopyridine, and carrying out a reaction to obtain the bis-n-alkyl dithio-n-alkanoic acid choline glycerophosphate.
Owner:SOUTHEAST UNIV
Method for purifying choline glycerophosphate
InactiveCN108329344ASimple purification processAvoid consumptionPhosphatide foodstuff compositionsPurification methodsRoom temperature
The invention discloses a method for purifying choline glycerophosphate. The method is characterized in that the method comprises the following steps: A. a choline glycerophosphate crude product is dissolved in a solvent, in order to obtain a reaction system dissolving liquid; B. calcium salt is added into reaction system dissolving liquid in the step A with stirring, in order to form a calcium salt compound precipitate of choline glycerophosphate; C. a precipitation reaction in the step B is stopped and a reaction solution is cooled to a room temperature, and filtering is carried out in orderto obtain a filter cake; the filter cake in the step C is placed in a baking oven for drying, in order to obtain calcium salt of choline glycerophosphate. Purified calcium salt can be used for removing calcium ions and corresponding complex anions by using an ion exchange resin exchange adsorption process, in order to obtain purified choline glycerophosphate without calcium ions. A nanofiltrationprocess can be used for removing calcium ions and complex anions, in order to obtain purified choline glycerophosphate. The method aims at overcoming deficiencies in the prior art, and the method forpurifying choline glycerophosphate with short time substantially reduces solvent consumption and power cost and reduces workload.
Owner:ZHONGSHAN BAILING BIOTECHNOLOGY CO LTD
Method Of Modifying Surface Of Material
ActiveUS20100285252A1Easy to adjustIncrease chancePretreated surfacesLoose filtering material filtersPhosphorylcholineChemical compound
A method for surface modification of a material by means of introducing the phosphorylcholine group represented by the following formula (1-1) onto the surface of the material by treating a material having amino groups with a chemical compound containing an aldehyde derivative obtained by the oxidative ring-opening reaction of glycerophosphorylcholine.The method of the present invention provides various materials such as medical materials having superior biocompatibility and hydrophilicity.
Owner:SHISEIDO CO LTD
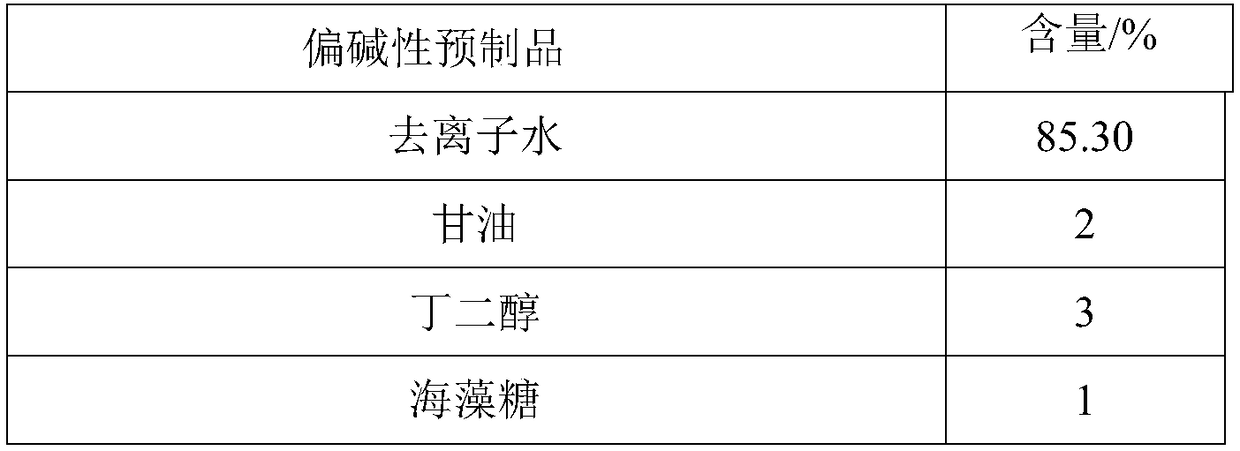
Two-agent carbonic acid foamed mask and preparation method and application method thereof
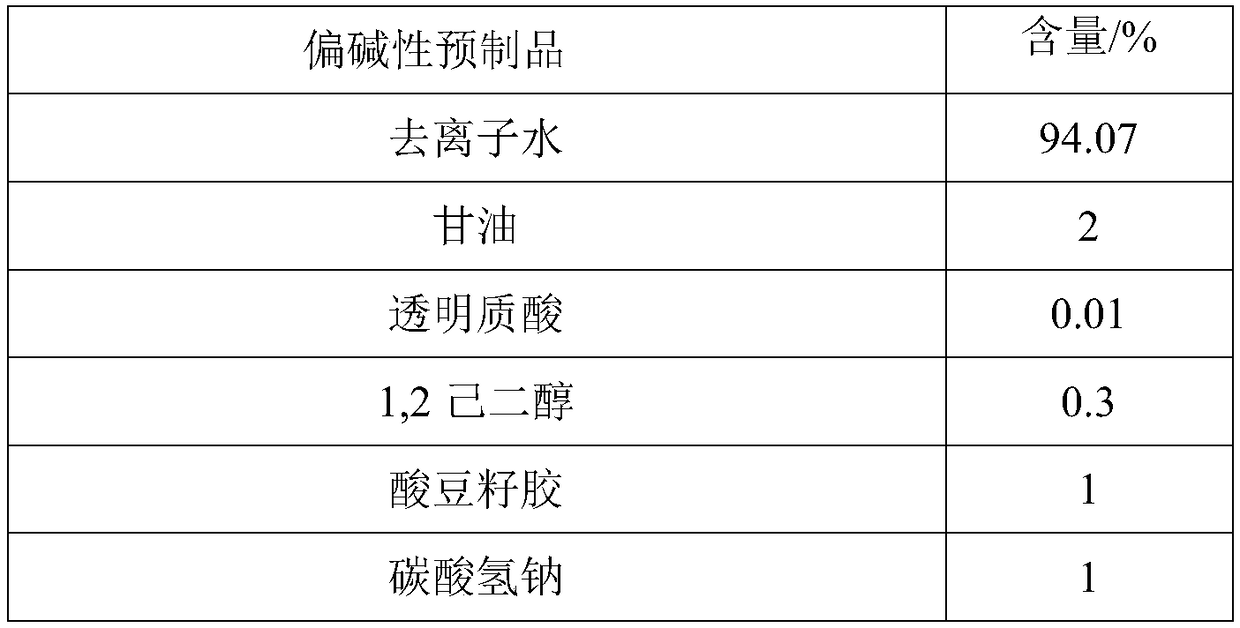
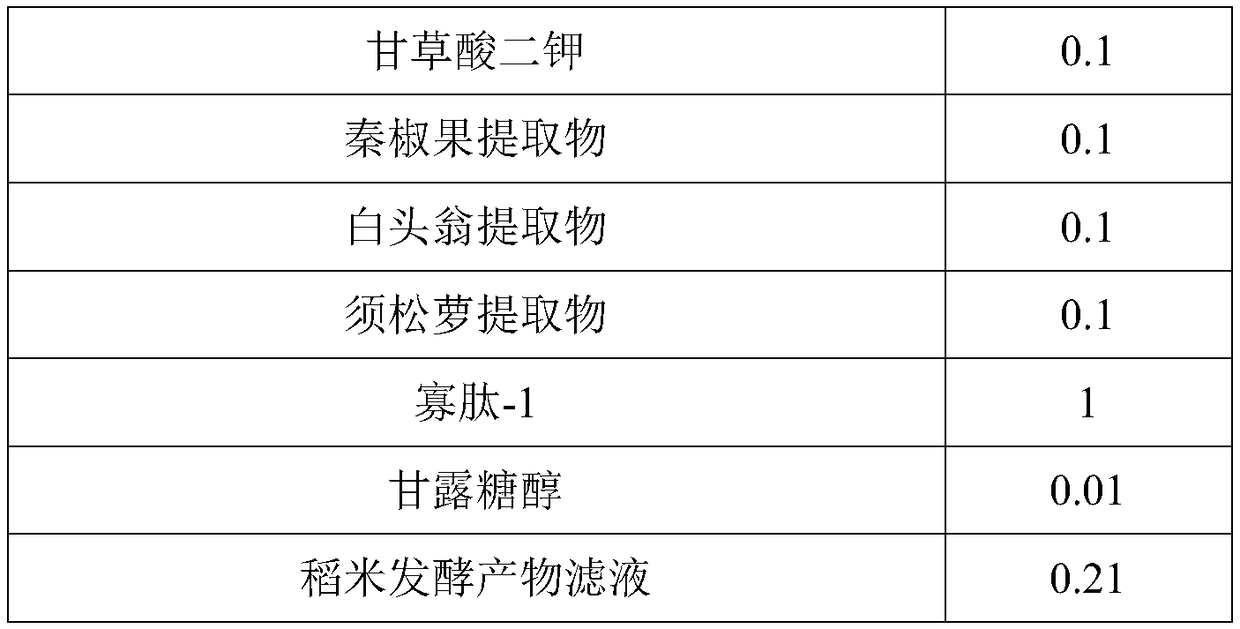
ActiveCN109077973AImprove antioxidant capacityImprove stabilityCosmetic preparationsToilet preparationsSodium bicarbonatePulsatilla Extract
The invention discloses two-agent carbonic acid foamed mask and a preparation method and application method thereof and is intended to provide a facial mask easy to clean, effective to foam, free of discoloring and good in whitening and moisturizing effect. The two-agent carbonic acid foamed mask comprises an acid prefabricated article and an alkaline prefabricated article; the acid prefabricatedarticle includes a polyol, xanthan gum, 1,2-hexanediol, citric acid, sodium citrate, polyglyceryl-10 laurate, inositol choline glycerophosphate, bisabolol, ginger root extract, opuntia streptacantha stem extract, Olea europaea fruit oil, and suitable deionized water; the alkaline prefabricated article includes a polyol, a humectant, 1,2-hexanediol, Tamarindus indica seed gum, sodium bicarbonate, dipotassium glycyrrhizinate, Zanthoxylum piperitum fruit extract, Chinese pulsatilla root extract, Usnea barbata extract, oligopeptide-1, mannitol, rice fermentation product filtrate, and suitable deionized water. The invention belongs to the technical field of cosmetics.
Owner:GUANGDONG BAWEI BIOLOGICAL TECH CO LTD
Nucleic acid vaccine composition comprising a lipid formulation, and method of increasing the potency of nucleic acid vaccines
PendingUS20200046830A1Enhance immune responseImprove the level ofSsRNA viruses negative-senseOrganic active ingredientsVaccine PotencyTGE VACCINE
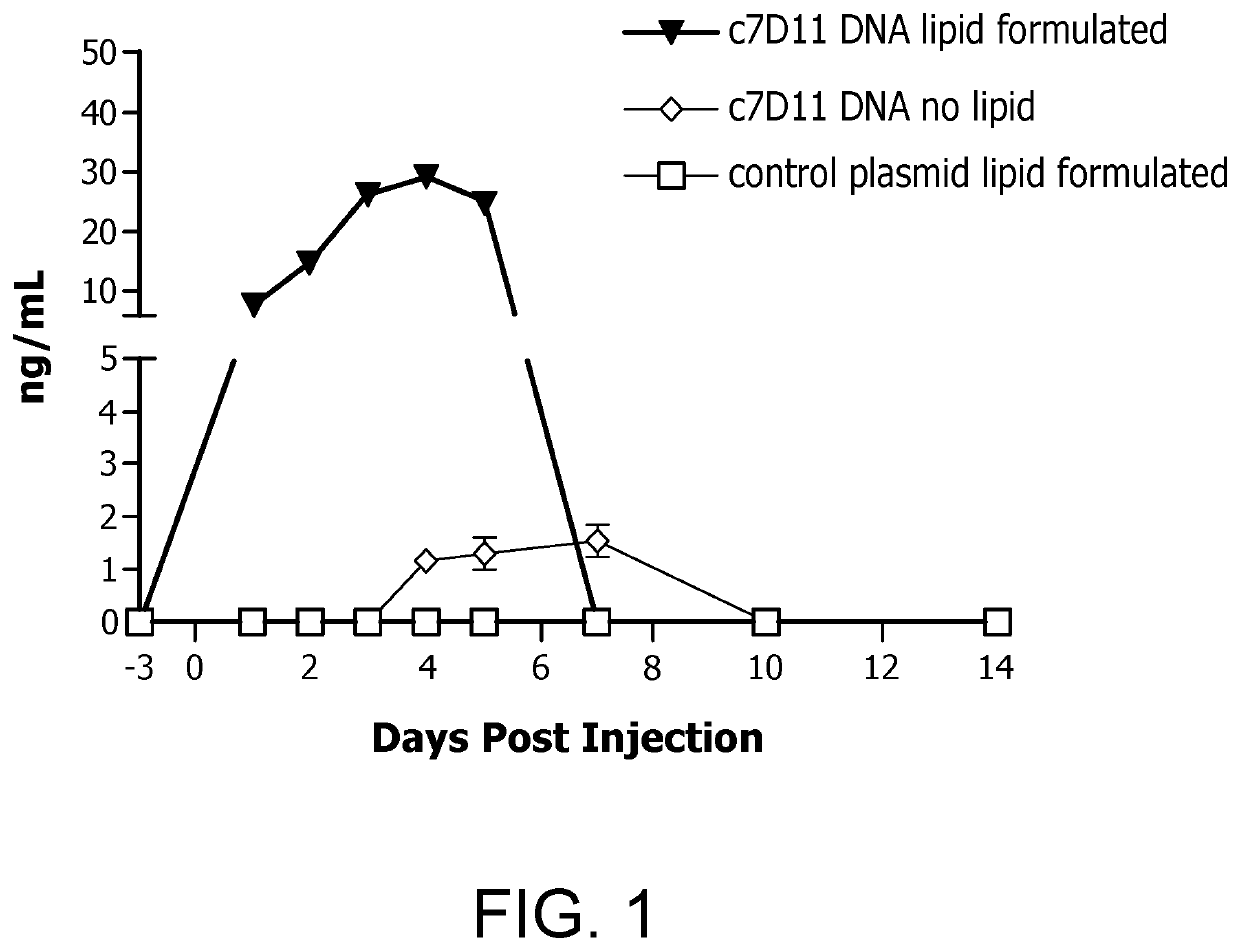
A nucleic acid vaccine composition comprising one or more of a plasmid-based nucleic acid vaccine and immunotherapy, as well as a lipid formulation, is provided. In addition, the present invention provides a method of enhancing the potency of plasmid-based DNA vaccines and immunotherapies, by formulating a vaccine and / or immunotherapy in a lipid formulation, which is stable when refrigerated or stored frozen, is then delivered to a vaccinee by either needle / syringe, jet injection, or microneedles. The lipid formulation of the present invention comprises one or more lipid excipients selected from 1,2-Distearoyl-sn-glycero-3-phosphocholine, Cholest-5-en-3β-ol, 1,2-Dimyristoyl-rac-glycero-3-methylpolyoxyethlene, and or more symmetric ionizable cationic lipids. The present invention increases vaccine potency dramatically. It was unexpectedly discovered that the level of immunogen, or immune response molecules, produced in vivo is increased (versus administering merely the vaccine or immunotherapy) and, in the case of a vaccine immunogen, the immune response is enhanced.
Owner:THE SEC OF THE ARMY +1
Dithiophospholipid compound and preparation method thereof
PendingCN113072578AReasonable structural designCan control targeted releasePharmaceutical non-active ingredientsPhosphorus organic compoundsDisulfide bondingWater methanol
The invention discloses a dithiophospholipid compound and a preparation method thereof. The preparation method comprises the following steps: weighing cystamine hydrochloride and triethylamine absolute methanol, adding di-tert-butyl dicarbonate, and reacting to obtain single protection cystamine; weighing fatty acid, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride and N-hydroxythiosuccinimide, dissolving in dichloromethane, adding mono-protected cystamine, mixing, dissolving reactants in dichloromethane, and adding trifluoroacetic acid to obtain a solid; dissolving the solid in dichloromethane, adding triethylamine and succinic anhydride, and reacting to obtain an intermediate product; and weighing the intermediate product according to the ratio, dissolving dicyclohexylcarbodiimide and 4-dimethylaminopyridine in dimethyl sulfoxide, activating, adding choline glycerophosphate, and reacting to obtain the target product. The structure of the dithiophospholipid compound provided by the invention contains a disulfide bond which is easily reduced by glutathione, so that phospholipid is broken in the presence of GSH, and the dithiophospholipid compound has a GSH response breaking function.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY
Method for synthesizing phosphatidylcholine by using solid-phase carrier
PendingCN114014889AIncrease the reactive surface areaEasy generationGroup 5/15 element organic compoundsPhosphatide foodstuff compositionsPharmaceutical AidsPhosphatidyl Cholines
The invention provides a method for artificially synthesizing phosphatidylcholine by using a solid-phase carrier, which comprises the following steps of: melting choline glycerophosphate, uniformly adsorbing the melted choline glycerophosphate with activated carbon, carrying out acylation reaction with fatty acid acyl chloride, and filtering and recrystallizing the obtained product to obtain high-purity phosphatidylcholine. The phosphatidylcholine prepared by the method disclosed by the invention can be used as a pharmaceutical adjuvant for various medicines such as injections, tablets and capsules.
Owner:江苏东南纳米材料有限公司
Glycerophosphoryl choline degradation or detection enzymologic method, product and application thereof
The present invention provides a glycerophosphoryl choline (GPC) degradation or detection enzymologic method, a product and application thereof. The method comprises simultaneous or successive contacting of glycerophosphoryl choline phosphodiesterase and alkaline phosphatase with GPC in a sample for degrading of the GPC; quantification of inorganic phosphorus in a reaction product; and determination of content of the glycerophosphoryl choline in the to-be-tested sample in accordance with the determined inorganic phosphorus content. The method can be used for high sensitivity and high accuracy quick and easy degradation or detection of the GPC, and has broad application prospects.
Owner:益海嘉里(连云港)生物科技有限公司
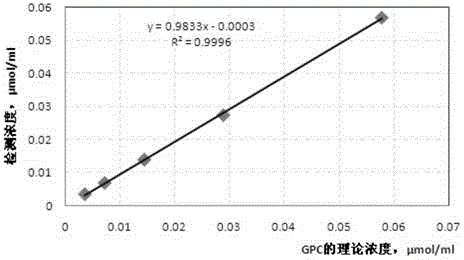
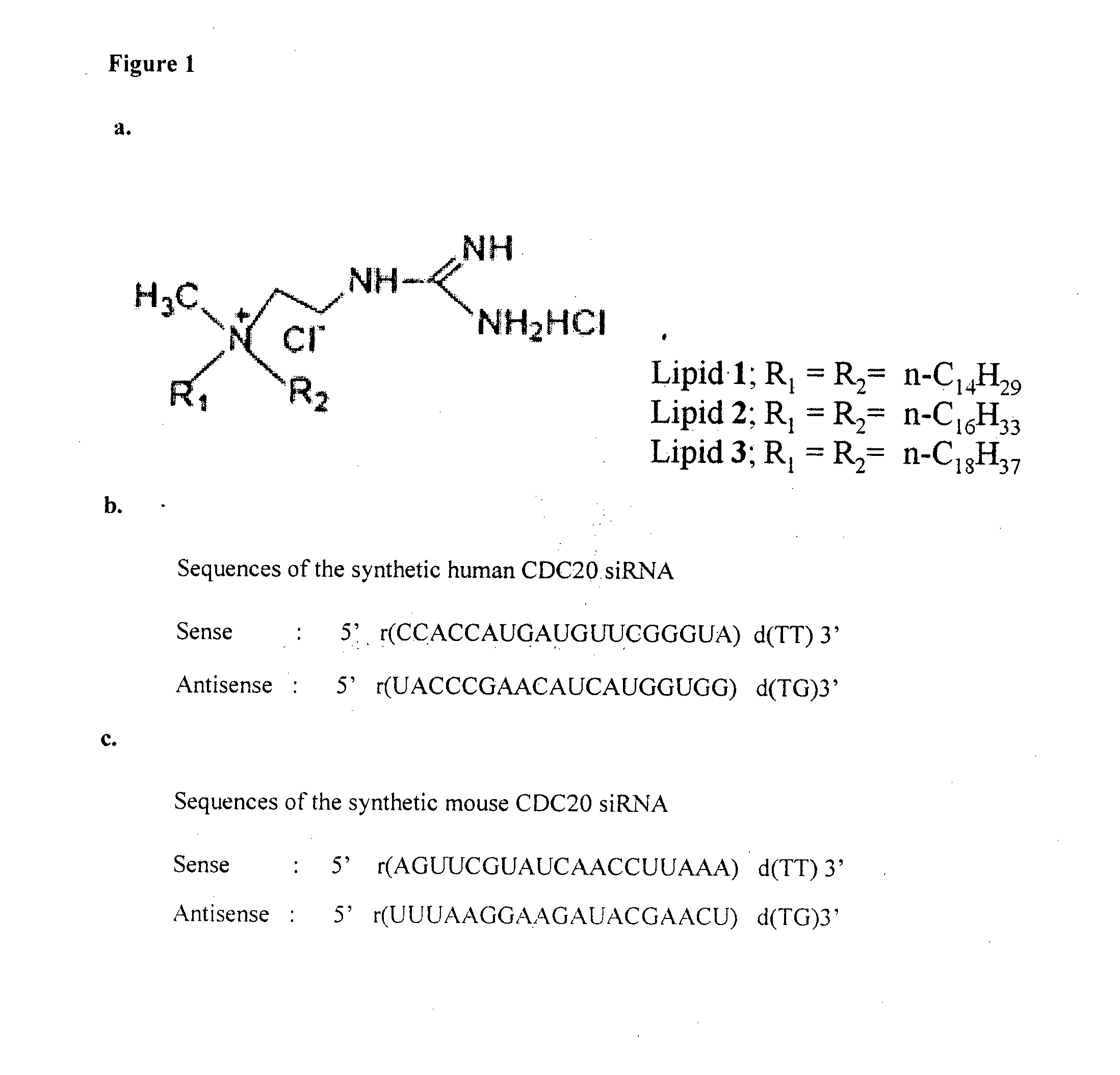
METHOD FOR INHIBITING TUMOR GROWTH THROUGH RNA-INTERFERENCE USING LIPOSOMALLY ASSOCIATED CDC20 siRNA
InactiveUS20160010088A1Improve efficiencyLow toxicitySpecial deliveryFermentationLipid formationMelanoma
Liposomal compositions comprising of liposomes of guanidinylated cationic amphiphiles as the main lipid and cholesterol / 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) / aminopropyl polyethyleneglycol carbamyl-distearoylphosphatidyl-ethanolamine (DSPE-peg-NH2) as co-lipids are described. These liposomal compositions containing encapsulated or electrostatically complexed therapeutic small interfering RNAs (siRNAs) against Cdc20, a key cell cycle regulator, inhibit solid tumor growth and melanoma tumor growth on lung in C57BL / 6J mice.
Owner:COUNCIL OF SCI & IND RES
Orally disintegrating tablet of choline glycerophosphate and preparation method thereof
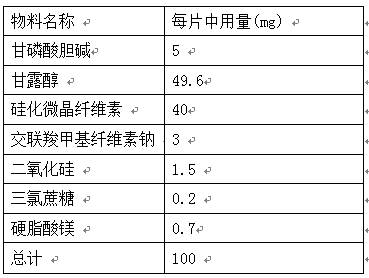
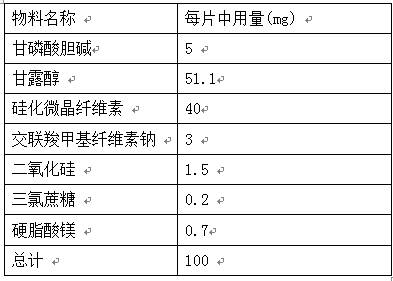
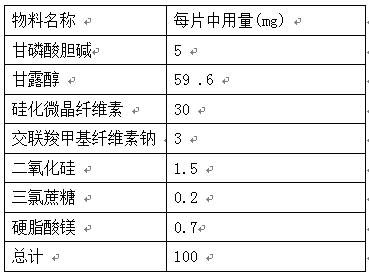
PendingCN112137971AGreat tasteGood taste acceptanceOrganic active ingredientsNervous disorderCross-linkCarboxymethyl cellulose
The invention provides an orally disintegrating tablet of choline glycerophosphate and preparation method thereof, which includes components of aripiprazole, silicified microcrystalline cellulose, cross linked sodium carboxymethyl cellulose, silica, fillers, flavoring agents and lubricating agents, and the aripiprazole is used as active component. On the basis of the formula, the product is prepared by powder direct pressing process. The product has better taste and more excellent long-term stability, so that the effectiveness and safety of the medicine are guaranteed.
Owner:BEIJING VENTUREPHARM BIOTECH
Composition for treating Alzheimer's disease as well as preparation method and application thereof
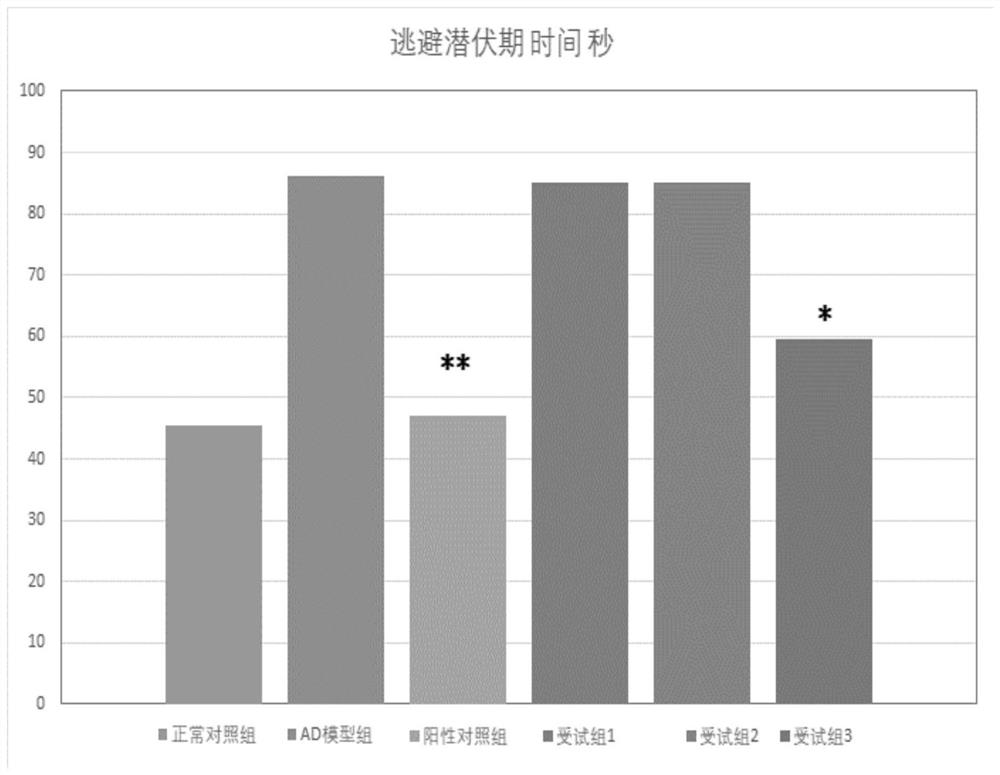
ActiveCN112716969AClear effectSecurity convincingOrganic active ingredientsNervous disorderDiseaseFood grade
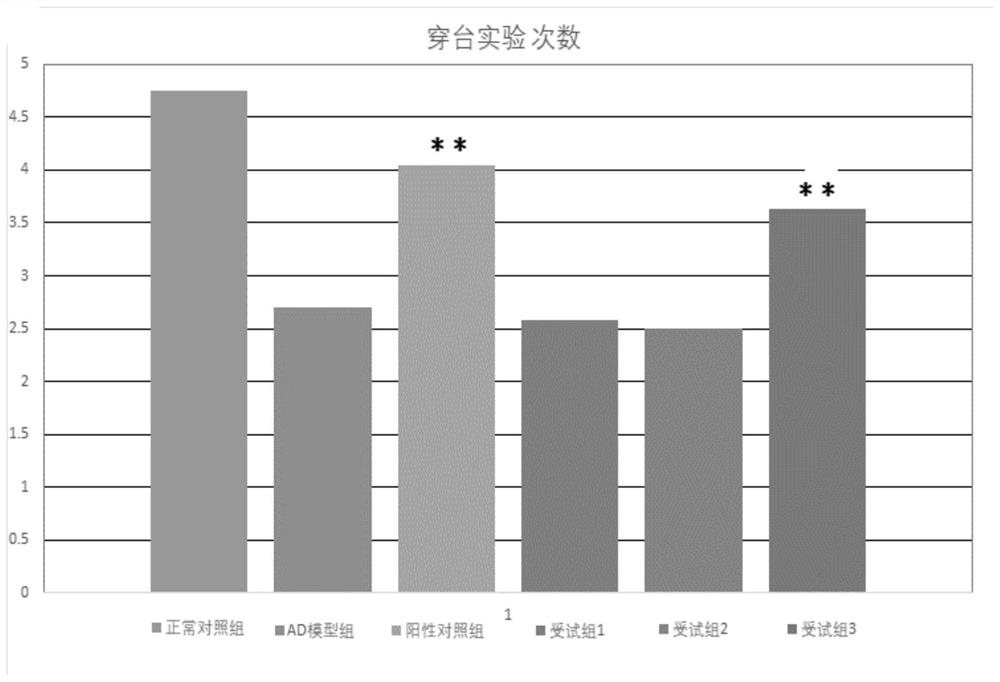
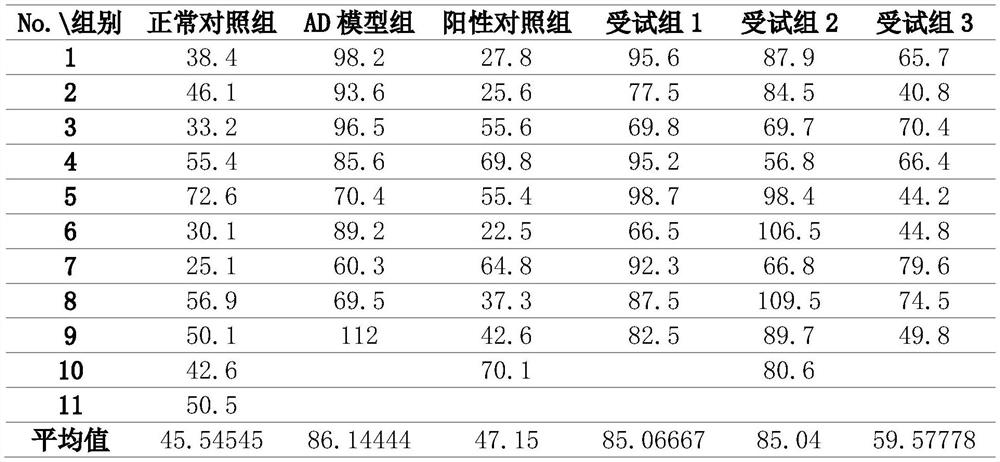
The present invention provides a composition for treating Alzheimer's disease, comprising beta nicotinamide mononucleotide (NMN) and L [alpha] glycerophosphorylcholine ([alpha] GPC), preferably in an amount of 3-9 mg / kg / d and in an amount of 8-20 mg / kg / d. In a classic Alzheimer's disease pharmacodynamic evaluation model Morris water maze test model, the composition provided by the invention has an obvious improvement effect on learning and memory behaviors of rats after artificial modeling. The composition is a food-grade raw material, is safe and reliable, and can be taken for a long time.
Owner:江苏恒正合生命科学有限公司
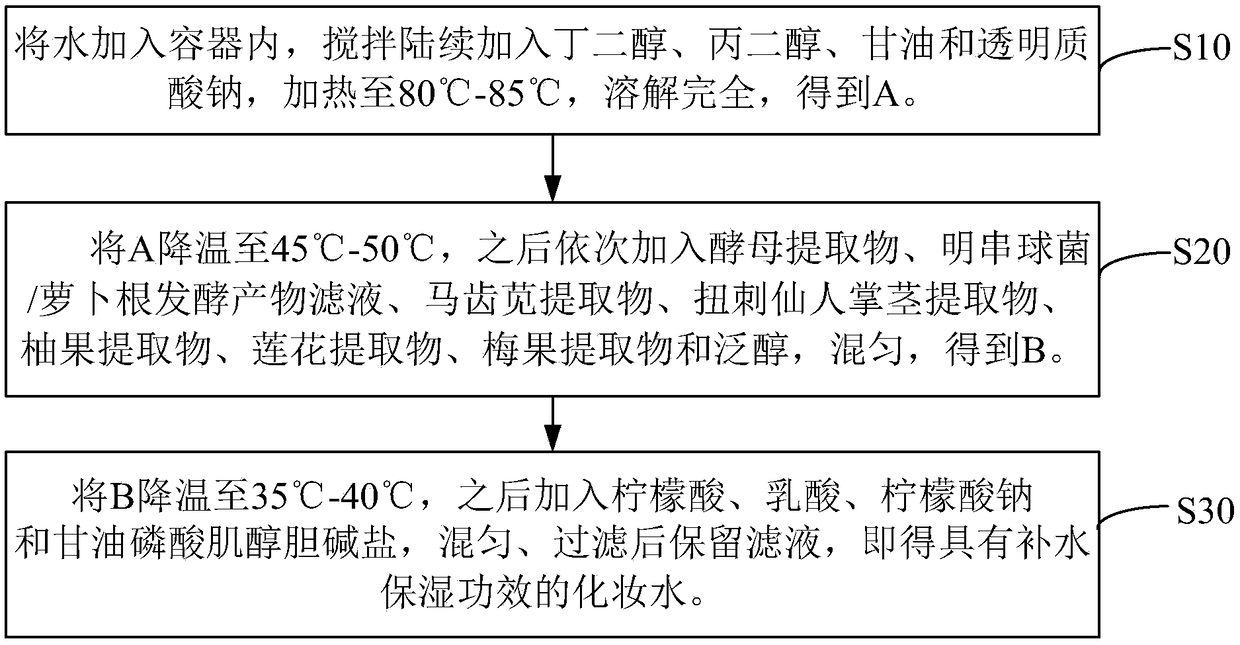
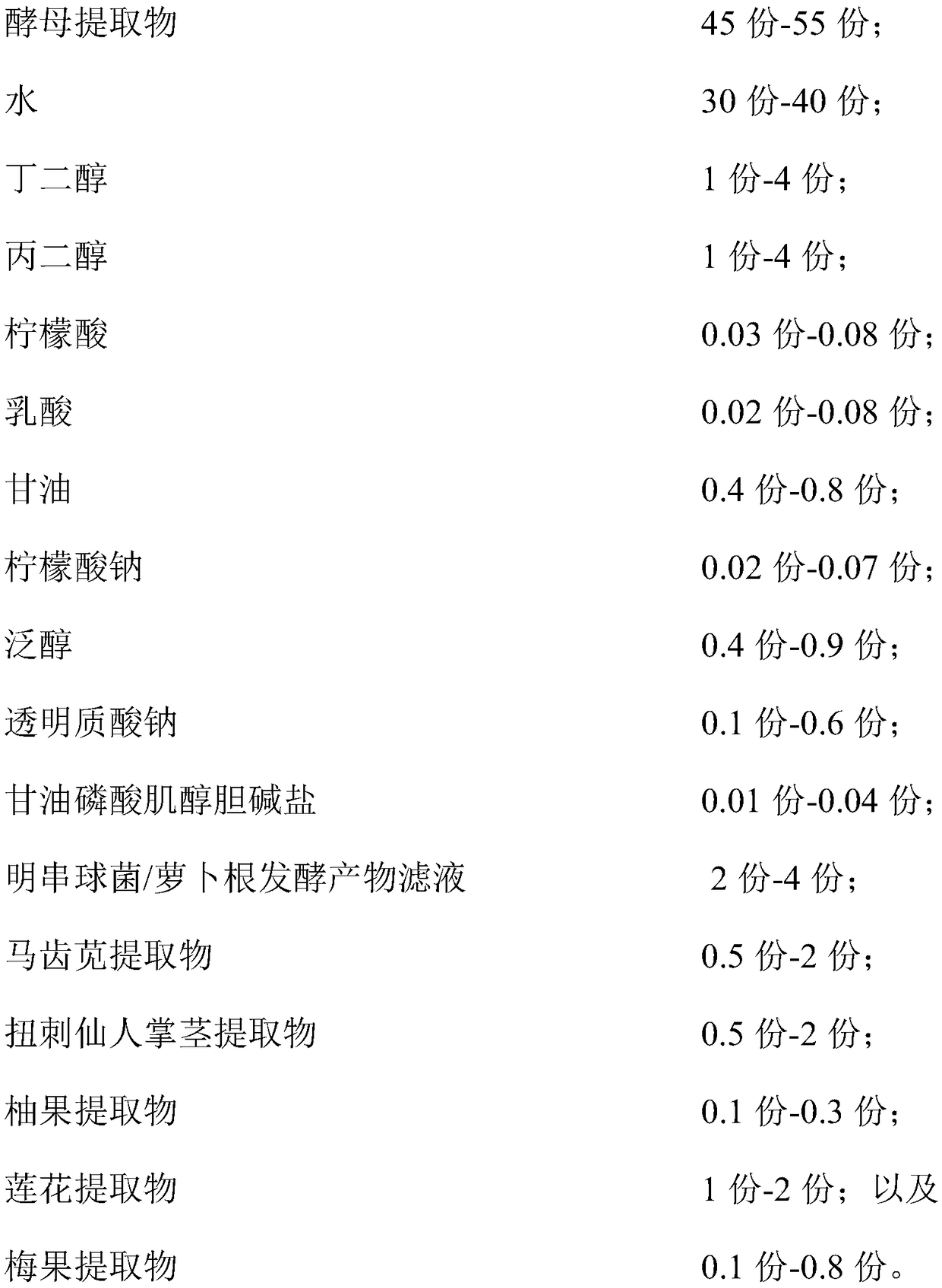
Hydrating composition, application of hydrating composition, hydrating toning lotion, and preparation method of the hydrating toning lotion
ActiveCN109350576AHas moisturizing effectPowerful moisturizing effectCosmetic preparationsToilet preparationsPurslane extractGlycerol
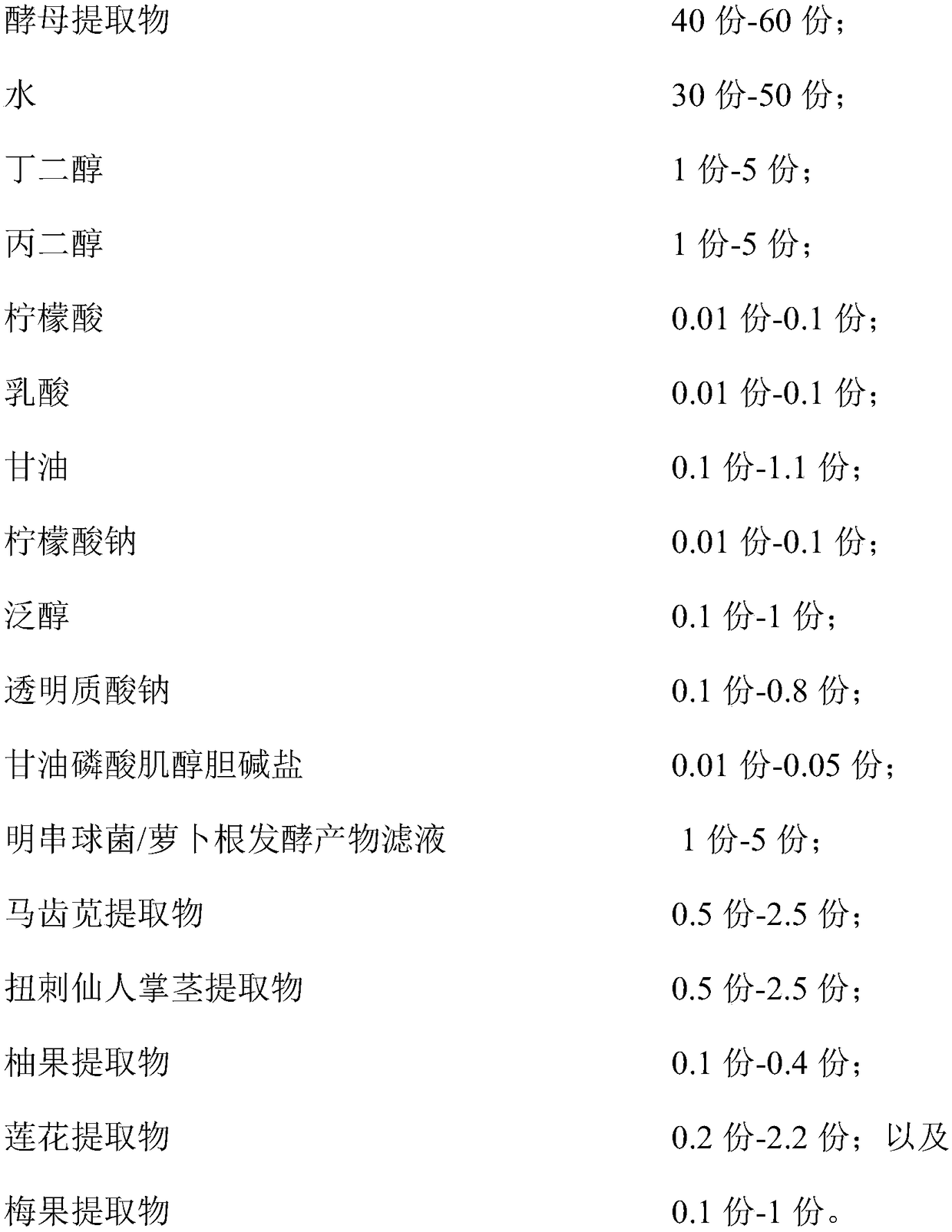
The invention relates to a hydrating composition, application of the hydrating composition, hydrating toning lotion, and a preparation method of the hydrating toning lotion. The hydrating compositioncomprises 40-60 parts of yeast extract, 30-50 parts of water, 1-5 parts of butanediol, 1-5 parts of propylene glycol, 0.01-0.1 part of citric acid, 0.01-0.1 part of lactic acid, 0.1-1.1 parts of glycerol, 0.01-0.1 part of sodium citrate, 0.1-1 part of panthenol, 0.1-0.8 part of sodium hyaluronate, 0.01-0.05 part of inositol choline glycerophosphate, 1-5 parts of Leuconostoc / radish root fermented product filtrate, 0.5-2.5 parts of purslane extract, 0.5-2.5 parts of Opuntia streptacantha stem extract, 0.1-0.4 part of grapefruit extract, 0.2-2.2 parts of lotus flower extract, and 0.1-1 part of Prunus mume fruit extract. The hydrating composition is good in safety and nonirritating, and has good hydrating effect.
Owner:广州柚子舍生物科技有限公司
Composition for treatment and/or prevention of alzheimer's disease
PendingCN110494144AEffective therapeuticEffective/or preventiveOrganic active ingredientsNervous disorderSide effectPhosphoric acid
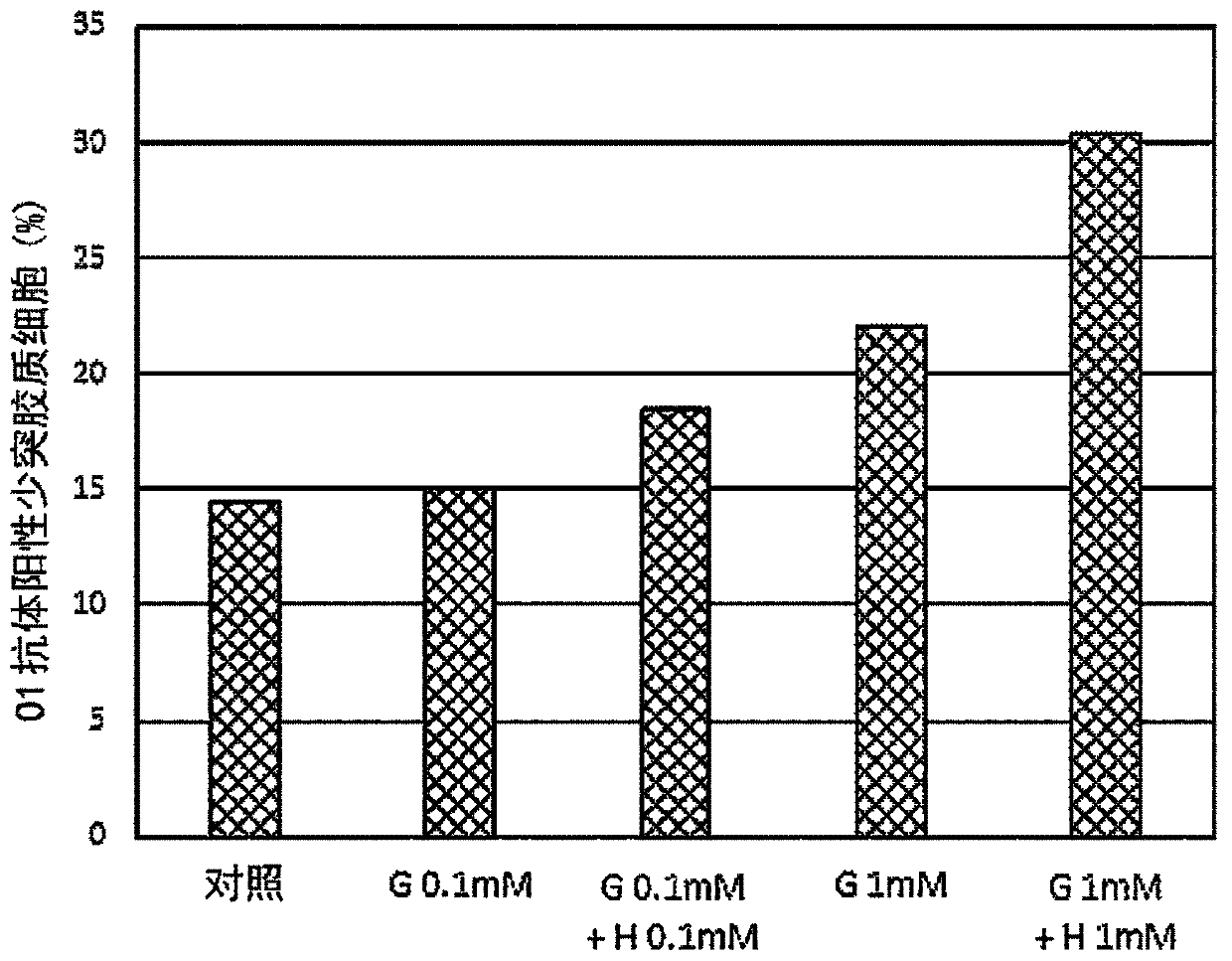
Provided is a composition for the treatment and / or prevention of Alzheimer's disease having an improved therapeutic or prophylactic effect together with reduced side effects. This composition for thetreatment and / or prevention of Alzheimer's disease contains at least one compound selected from the group consisting of glycerophosphocholine (G) and pharmaceutically acceptable salts thereof as a first active ingredient and at least one compound selected from the group consisting of herperidin (H), narirutin (N), and pharmaceutically acceptable salts thereof as a second active ingredient. This composition promotes remyelination, promotes the activity of alpha-secretase, and also suppresses the expression of beta-secretase.
Owner:GLOVIA
Composition having function of inhibiting fat formation and antioxidant activity and application of composition
InactiveCN110812383ALose weightImprove antioxidant capacityHydroxy compound active ingredientsMetabolism disorderRHODIOLA ROSEA ROOTGlycerol
The invention provides a composition having a function of inhibiting fat formation and antioxidant activity. The composition includes an effective dose of rhodiola rosea extract, glycerophosphoryl choline and a pharmaceutically acceptable carrier or pharmaceutically acceptable salt. Animal experiments confirm that a mixture of the rhodiola rosea extract and the glycerophosphoryl choline can effectively reduce body fat in animals and improve the antioxidant effect.
Owner:黄福星
Tenofovir and preparation technology thereof
InactiveCN110101682ASimple processIncrease acquisition rateOrganic active ingredientsGroup 5/15 element organic compoundsMethyl lactateSurface-active agents
The invention discloses tenofovir and a preparation technology thereof, and relates to the technical field of the tenofovir. The tenofovir comprises a capsule wall material and a capsule core, whereinthe capsule core is prepared from, by weight, 23-25 parts of R-methyl lactate, 7-9 parts of adenine, 13-15 parts of diisopropyl-p-tosyloxymethylphosphonate and 3-5 parts of sodium borohydride; the capsule wall material is prepared from, by weight, 17-19 parts of choline glycerophosphate, 17-19 parts of palmitic acid, 2-3 parts of sodium hydroxide, 4-6 parts of ethyldiisopropylamine, 6-9 parts ofparaformaldehyde, 4-7 parts of acetic acid, 7-9 parts of polyacrylic resin, 12-19 parts of deionized water, 8-12 parts of absolute ethyl alcohol, 3-7 parts of a surface active agent, 4-6 parts of a water-soluble dispersing agent and 5-10 parts of a solvent. The tenofovir prepared by using the technology is composed of the capsule core and the capsule wall material, the physical properties of the tenofovir are improved, the tenofovir is easily absorbed by the human body, and therefore, the pharmaceutical effect of the tenofovir is improved.
Owner:南京望知星医药科技有限公司
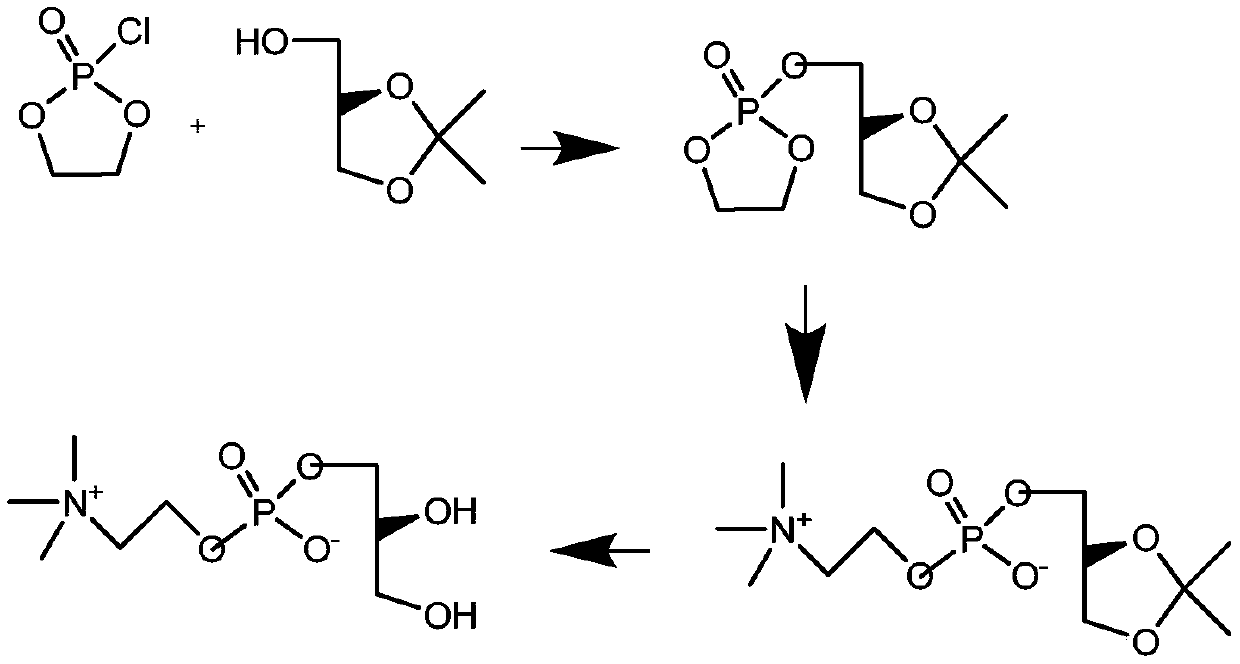
A kind of preparation method of l-alpha-glycerophosphocholine
ActiveCN108191908BEmission reductionHigh purityPhosphorus organic compoundsAsymmetric synthesesPhosphoric Acid EstersPhosphate
The invention relates to a preparation method of L-alpha-glycerophosphoryl choline. The method comprises the steps of preparing (R)-3-chlorine-1,2-propylene glycol as an initial raw material; preparing (R)-glycerophosphate with calcium phosphate metal salt; then carrying out a reaction with dibromoethane to obtain (R)-3-glyceryl cyclophosphate; finally carrying out a reaction with trimethylamine in an open-loop reaction to obtain L-alpha-glycerophosphoryl choline. With the adoption of the method, the problem of wastewater pollution caused by choline chloride phosphate calcium salt or potassiumsalt is avoided, and the link of removing chloridion of a finished product through ion-exchange columns can be eliminated; the product purity is high; the yield is high; the method is applicable to industrial production and has a good application prospect.
Owner:SHANGHAI KEYI BIOLOGICAL MEDICINE CO LTD
Composition with fat formation restraining and antioxidative activity promoting functions and treating method thereof
ActiveUS20200129576A1Antioxidative activity is improvedInhibition formationHydroxy compound active ingredientsMetabolism disorderPhosphoryl cholineGlycerol
A composition for promoting antioxidative activity includes an effective dose of Rhodiola extract, an alpha-Glycerophosphocholine (alpha-GPC), and a pharmaceutically acceptable vehicle or salt thereof. Based on animal experiments, the combination of rhodiola extract and alpha-Glycerophosphocholine provides high antioxidative activity.
Owner:HUANG FU HSING
Bicontinuous phase moistening and nourishing makeup removing gel and preparation method thereof
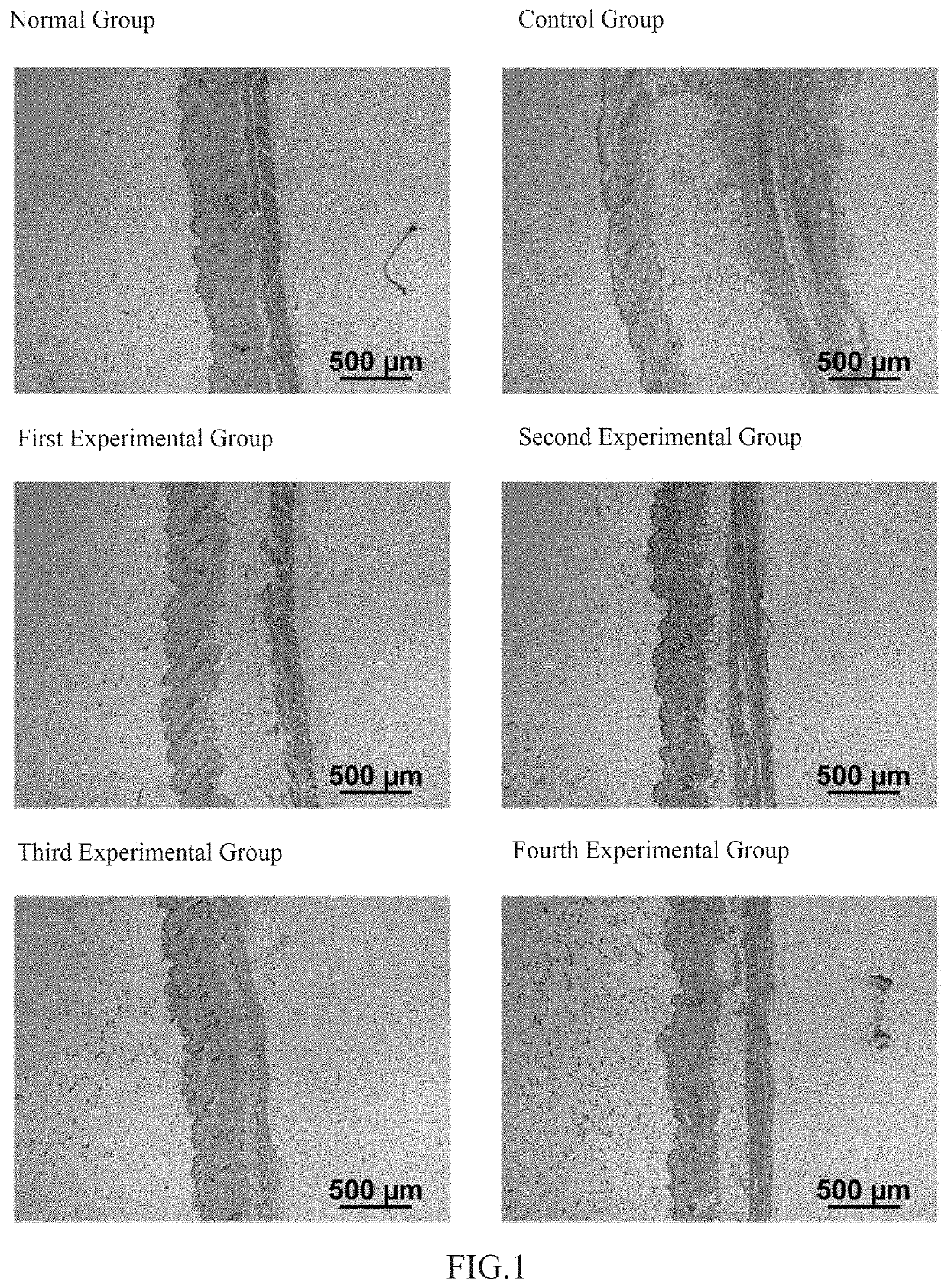
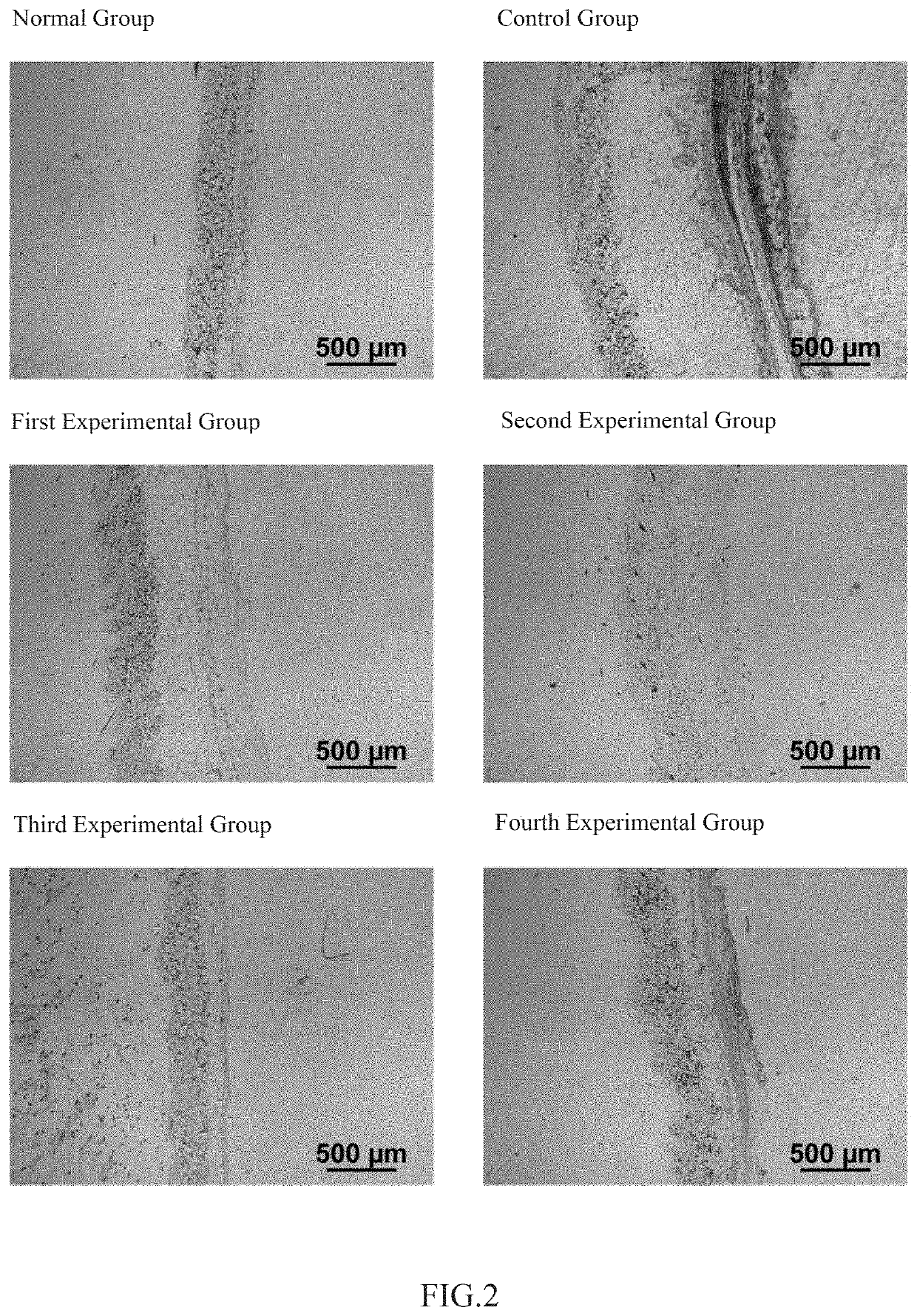
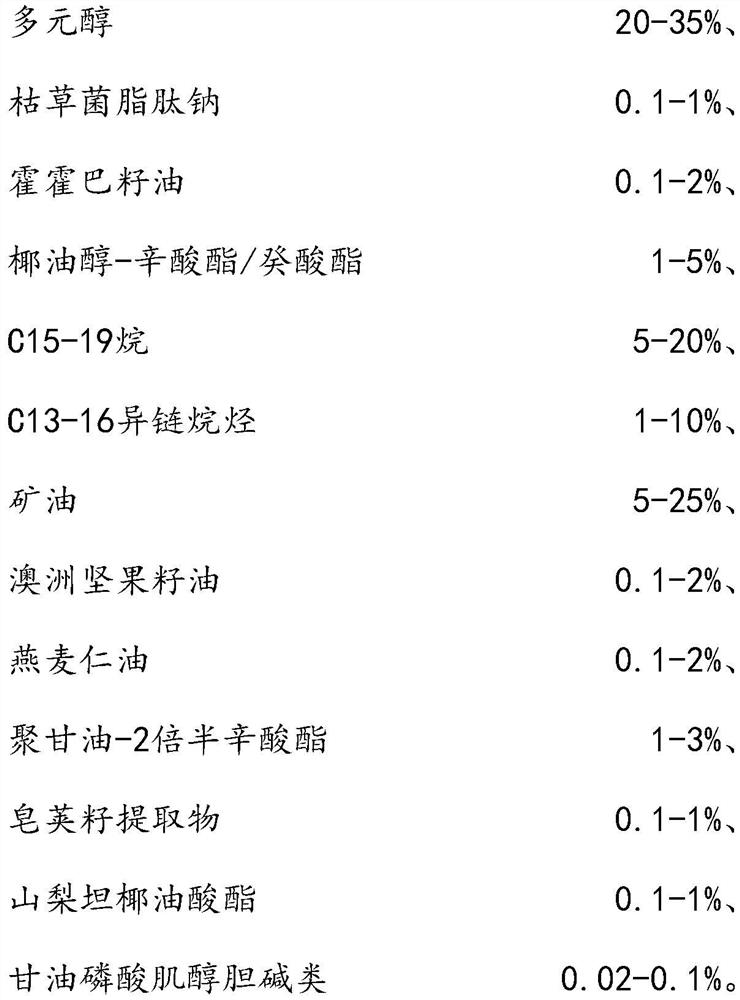
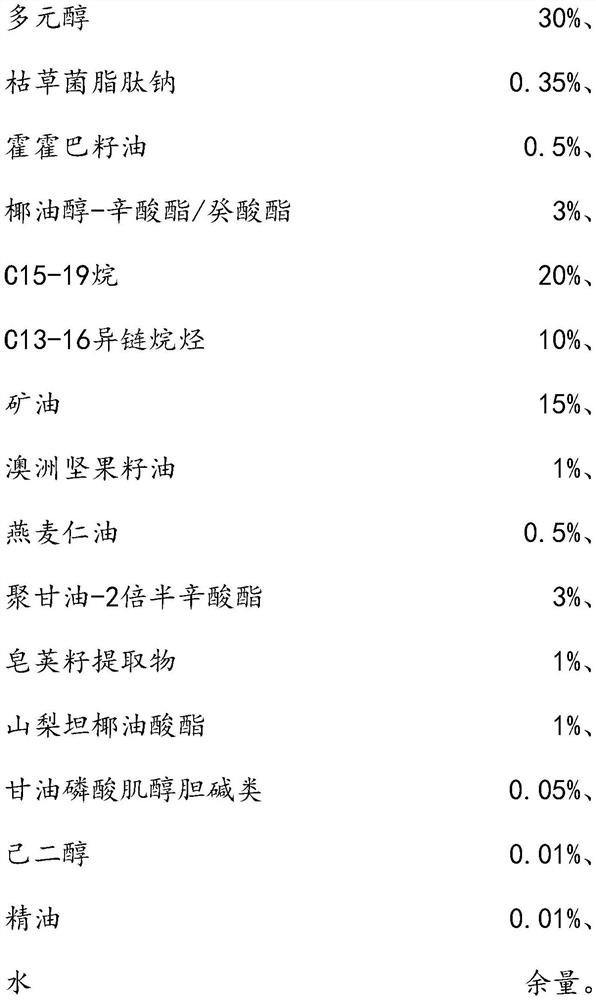
The invention relates to the technical field of daily chemicals, and aims to provide a bicontinuous phase moistening and nourishing makeup removing gel and a preparation method thereof. The preparation raw materials comprise a D-phase component, an auxiliary component and the balance of water, wherein the phase D is prepared from polyhydric alcohol, sodium surfactin, simmondsia chinensis seed oil coconut oil alcohol-octoate / caprate, C15-19 alkane, C13-16 isoparaffin, mineral oil, macadamia nut seed oil, oat kernel oil, polyglycerol-2 sesquioctoate, a gleditsia sinensis seed extract, sorbitan cocoate and inositol choline glycerophosphate. The whole system forms a transparent bicontinuous transparent gel system, is different from traditional cleansing gel, has the advantages of cleansing water and cleansing oil, can effectively remove facial dirt and makeup, can nourish and repair the skin, and achieves the mild washing, cleansing and caring three-in-one effect.
Owner:羽楠(广州)化妆品有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com