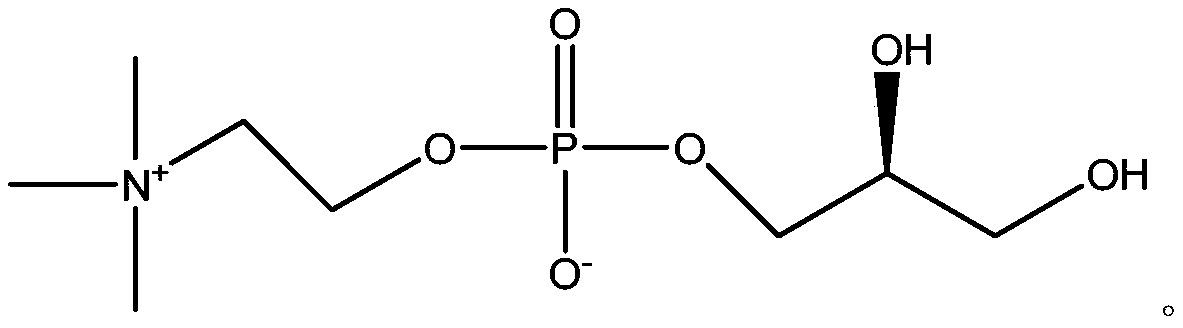
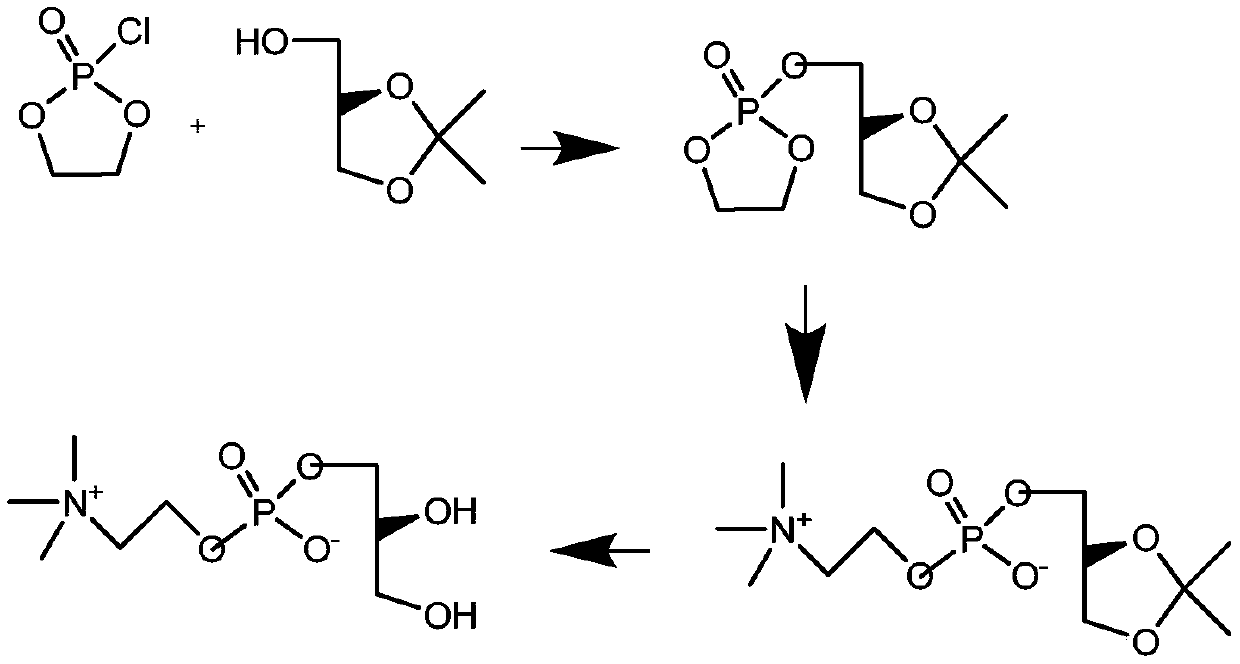
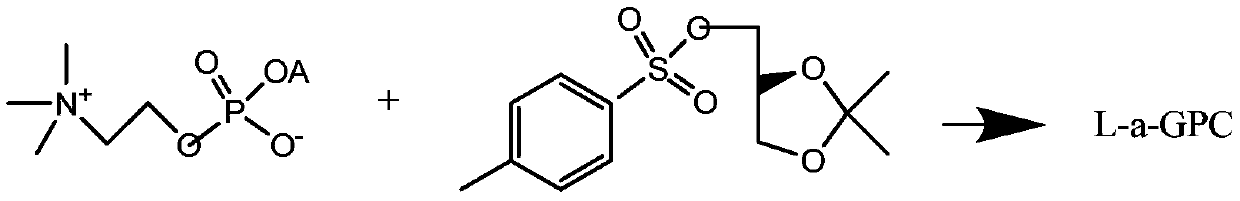
A kind of preparation method of l-alpha-glycerophosphocholine
A technology of glycerophosphocholine and glycerophosphate, which is applied in organic chemical methods, chemical instruments and methods, phosphorus organic compounds, etc., can solve pollution and other problems, and achieve the effects of reducing production costs, good application prospects, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of (R)-glycerophosphate
[0043]
[0044] Take 212.3 g (1 mol) of potassium phosphate and 1000 ml of purified water, stir to dissolve, heat to 45-50°C, and add 110 g (1.0 mol) of (R)-3-chloro-1,2-propanediol dropwise. After dropping, keep warm for 2-3 hours. Cool to room temperature, and adjust the pH value to 4-5 with dilute hydrochloric acid. The water was distilled off to dryness. Add 500 ml of ethanol and heat to reflux, then heat filter to remove inorganic salts. Ethanol was distilled to dryness to obtain 149.53 g of the product, with a yield of 87.43%.
Embodiment 2
[0046] Preparation of (R)-glycerophosphate
[0047]
[0048]Take 164 g (1 mol) of sodium phosphate and 1000 ml of purified water, stir to dissolve, heat to 45-50°C, and add 100 g (0.9 mol) of (R)-3-chloro-1,2-propanediol dropwise. After dropping, keep warm for 2-3 hours. Cool to room temperature, and adjust the pH value to 4-5 with dilute hydrochloric acid. The water was distilled off to dryness. Add 500 ml of ethanol and heat to reflux, then heat filter to remove inorganic salts. Ethanol was distilled to dryness to obtain 130.86 g of the product, with a yield of 83.23%.
Embodiment 3
[0050] Preparation of (R)-3-Glyceryl Cycloethyl Phosphate
[0051]
[0052] Take 172 grams (1.0 moles) of (R)-glycerophosphate of Example 1, 1500 milliliters of absolute ethanol, stir and dissolve at room temperature, add 276.5 grams (2.0 moles) of anhydrous potassium carbonate, benzyltriethylammonium bromide 5 grams (0.02 moles; act as a phase transfer catalyst), 188 grams (1.0 moles) of dibromoethane, heated to reflux for 6 hours, then added 94 grams of dibromoethane (0.5 moles), and continued to reflux for 3 hours . Cool to room temperature and filter. The solvent was distilled to dryness. Add 500 ml of water and extract with ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, and filtered. The solvent was distilled to dryness to obtain 130.02 g of a white solid product with a yield of 66.17%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com