Recombinant adenovirus for expressing porcine circovirus type-3 ORF2 gene and preparation method and application of recombinant adenovirus
A technology of recombinant adenovirus and gene fragments, which is applied in the field of porcine circovirus type 3 adenovirus vector vaccine, achieving the effects of high yield, low dosage, high immunogenicity and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Construction of a recombinant adenovirus transfer vector carrying the Cap protein coding gene of porcine circovirus type 3
[0042] 1. Codon optimization and synthesis of the Cap protein coding gene of PCV3
[0043] With reference to the sequence of the Cap protein coding gene (ORF2 gene) of PCV3 in the database, in order to achieve high-efficiency expression of the Cap protein, the present invention first optimizes the ORF2 gene sequence according to the codon preference of the adenovirus expression system, and designs different The codon optimization sequence of the codon, and the preliminary detection of its expression level. After comparison and screening, the present invention found that the expression level of the coding sequence obtained by different degrees of codon optimization is quite different, and the codon optimization position and degree of optimization and expression There is no clear rule between levels. For example, the expression level of the cod...
Embodiment 2
[0064] Example 2 Construction of recombinant adenovirus Ad-PCV3 Cap
[0065] 1. Construction of recombinant adenovirus plasmid pAd-PCV3 Cap
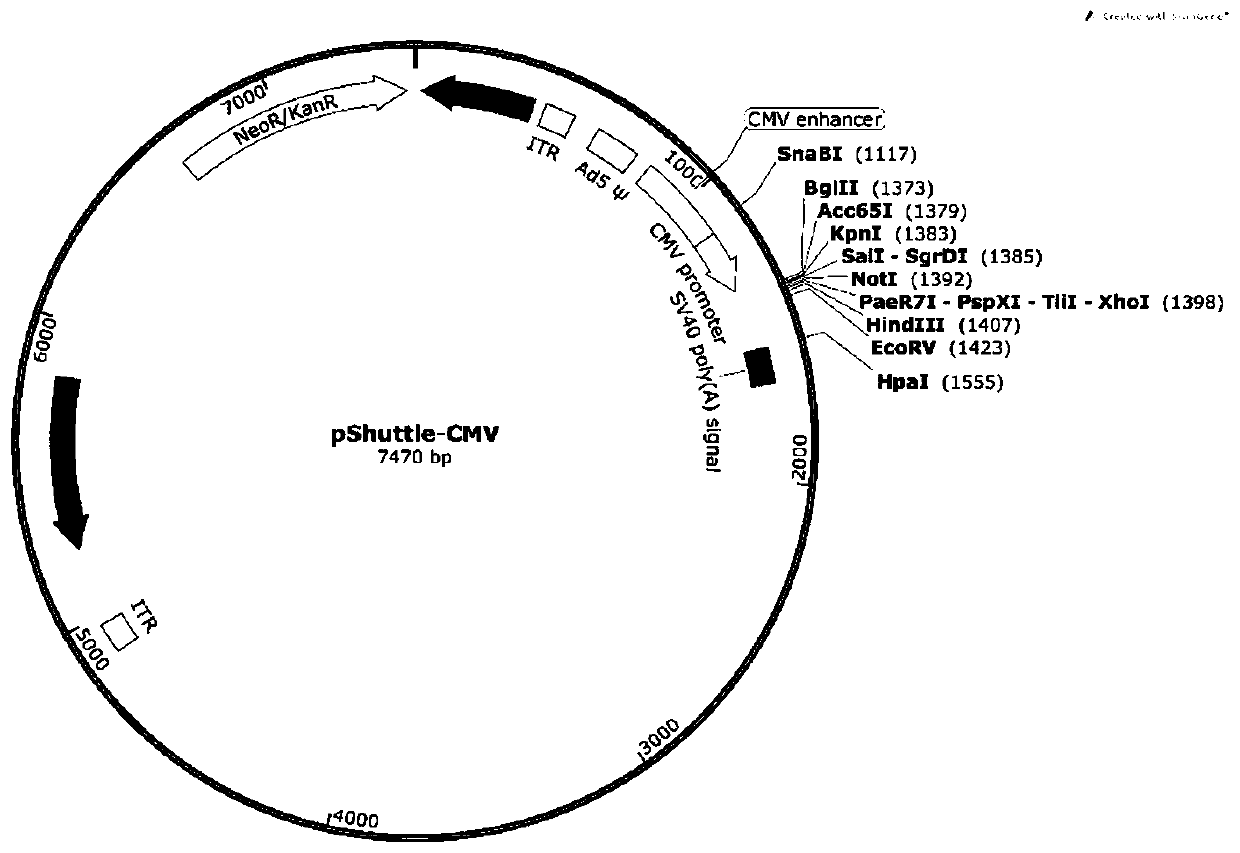
[0066] The recombinant plasmids pShuttle-CMV-Cap-1, pShuttle-CMV-Cap-2 and pShuttle-CMV-Cap-3 were linearized with PmeI, and the digested products were recovered and transformed into E.coli containing the backbone vector pAdEasy-1 BJ5183 competent cells. Pick the positive clones containing the recombinant adenovirus plasmids pAd-PCV3 Cap-1, pAd-PCV3 Cap-2 and pAd-PCV3 Cap-3, and extract the plasmids after shaking. The recombinant adenovirus plasmids pAd-PCV3 Cap-1, pAd-PCV3 Cap-2 and pAd-PCV3 Cap-3 were digested and identified with PacⅠ. The identification results are as follows image 3 Shown, and sent for sequencing verification.
[0067] Obtain a large number of pAd-PCV3 Cap recombinant plasmids: Take 2μl of recombinant transfer plasmids pAd-PCV3 Cap-1, pAd-PCV3 Cap-2, and pAd-PCV3 Cap-3 into 100μl of E. coli competent DH10B. After 30 minu...
Embodiment 3
[0075] Example 3 Evaluation of safety and immunogenicity of adenovirus Ad-PCV3 Cap vector vaccine
[0076] 1. Safety evaluation of adenovirus Ad-PCV3 Cap vector vaccine in mice
[0077] Purchase 10 female Balb / C mice of 16-18g and divide them into two groups A and B with 5 mice in each group. Each mouse in group A was injected subcutaneously with 10 8 The recombinant adenovirus Ad-PCV3 Cap-1 prepared in Example 2 of TCID50; each mouse in group B was injected subcutaneously with 0.1mL sterile PBS and observed for 14 days. The two groups of mice were in the same state and there was no abnormal reaction. This result It shows that the adenovirus vector vaccine prepared from the recombinant adenovirus of Example 2 is safe for mice.
[0078] 2. The adenovirus Ad-PCV3 Cap vector vaccine was evaluated for immunogenicity in mice
[0079] Purchase 10 female Balb / C mice aged 6-8 weeks and randomly divide them into two groups A and B, with 5 mice in each group. Each mouse in group A was injecte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com