Perillyl alcohol-3-bromopyruvate conjugates and methods of treating cancer
A technology of bromopyruvate and perillyl alcohol, applied in the field of cancer treatment, can solve the problems of patient death and low anticancer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
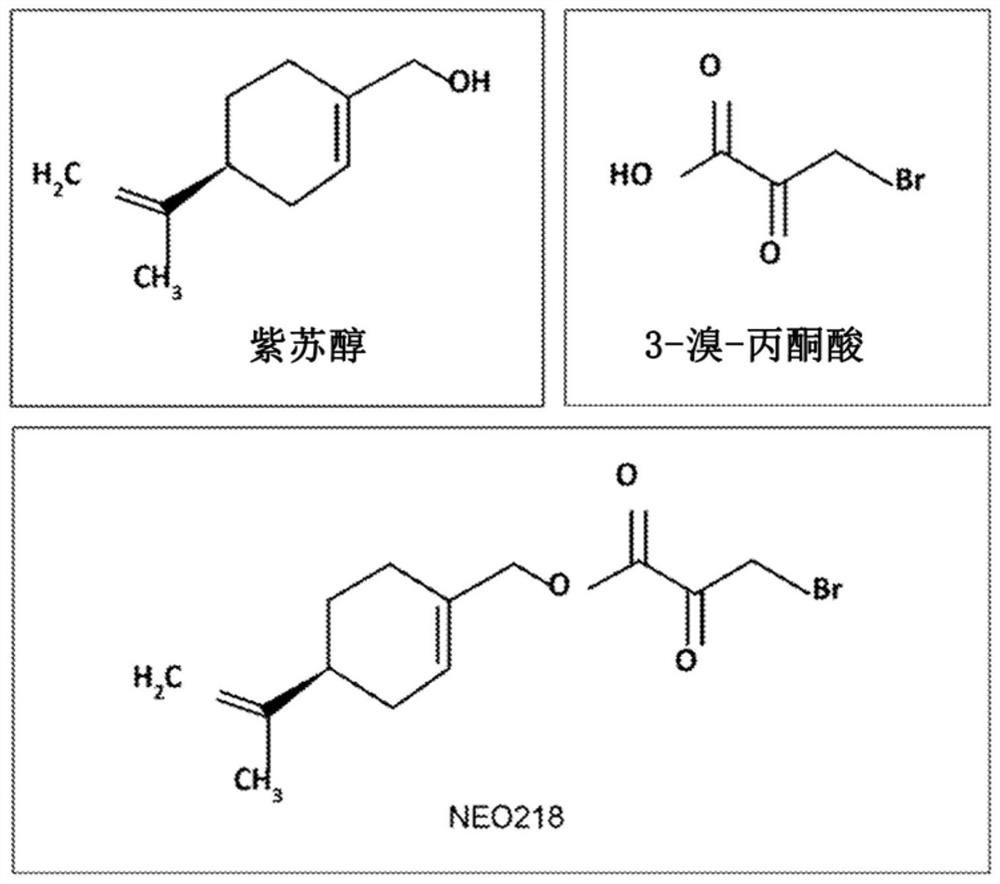
[0073] Example 1: Preparation of POH-bromopyruvate (3-bromo-2-oxoylidene-propionic acid 4-isopropenyl-cyclohex-1-enylmethyl ester);
[0074]
[0075] 1,1-Dichlorodimethyl ether (2.5 g, 21.74 mmol) was slowly added to solid bromopyruvic acid (1) while keeping the temperature below 20°C. The resulting slurry was slowly heated to 50 °C and stirred for 2.5 h. The clear liquid was cooled and the excess dichlorodimethyl ether was concentrated in vacuo to obtain 3-bromopyruvyl chloride (2) in greater than 95% yield.
[0076] 3-Bromopyruvyl chloride (2.0 g, 10.78 mmol) was added to a low temperature mixture of perillyl alcohol (3) (1.5 g, 9.85 mmol), sodium bicarbonate (11.90 mmol) and n-heptane (180 mL) while maintaining the temperature at below 10°C. The mixture was stirred at 10 °C for 20 min and then allowed to warm to room temperature. The reaction mixture was stirred for 18 h and quenched with water (75 mL). The organic layer was separated and washed with brine (75 mL) an...
Embodiment 2
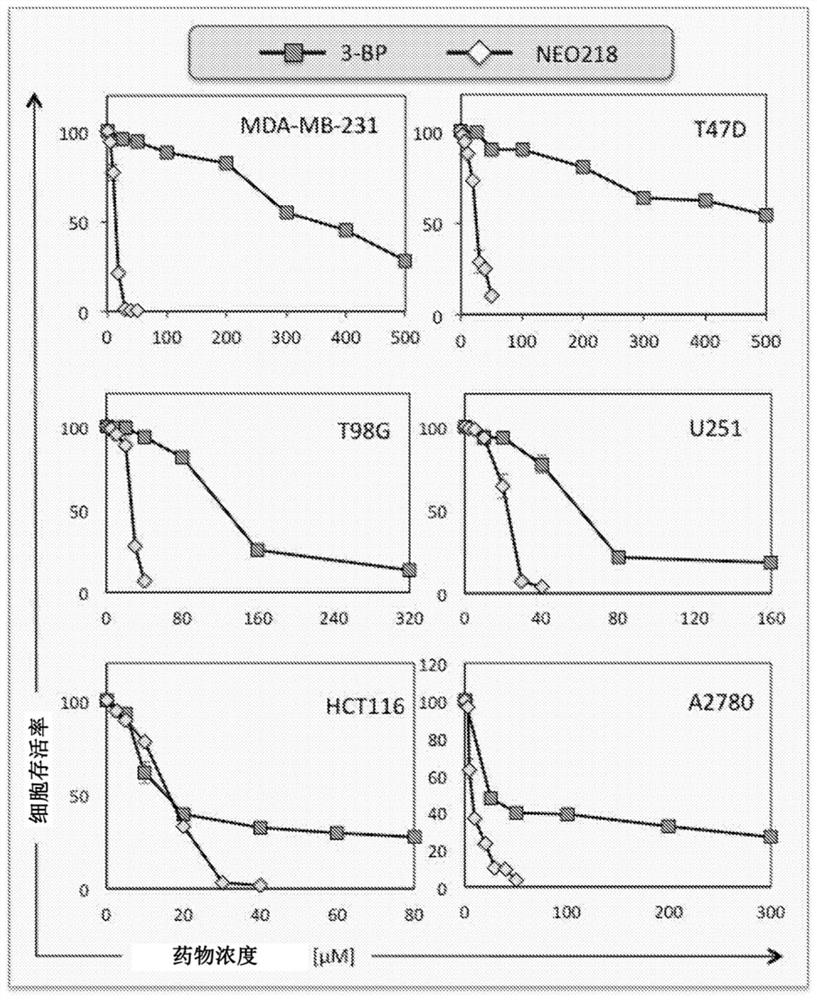
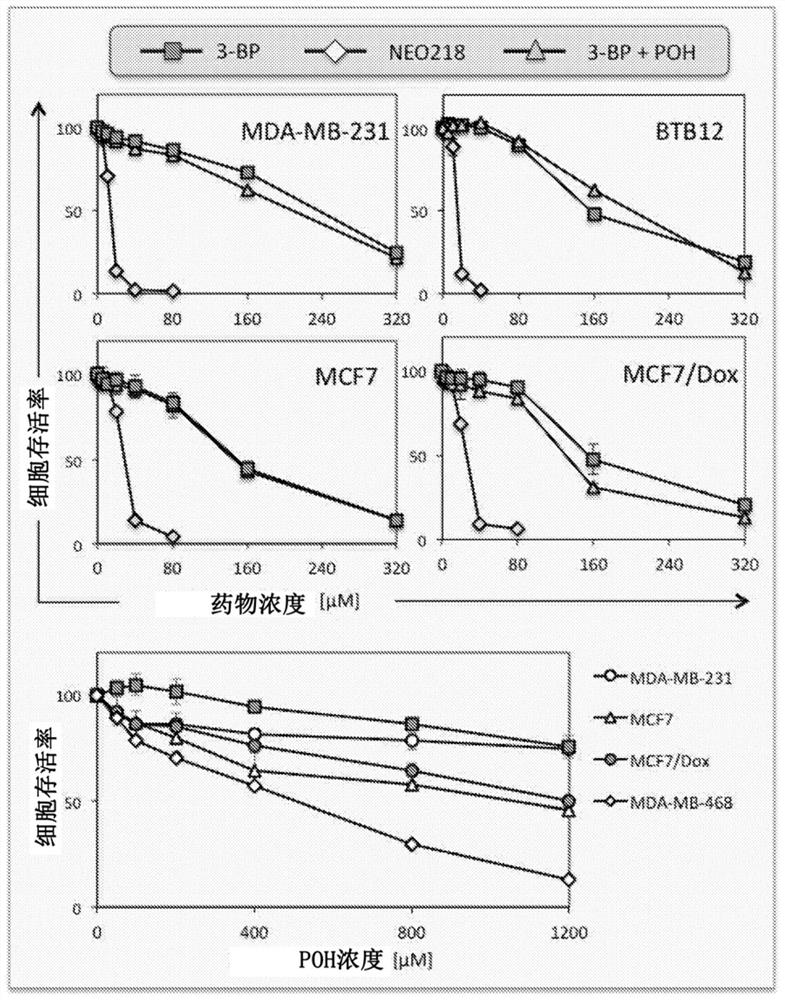
[0224] Example 2 Perillyl alcohol-conjugated 3-bromopyruvate analogs, cellular uptake is independent of monocarboxylic acid transporter 1 and is active in 3-BP-resistant tumor cells
[0225] Abbreviations: 3-BP: 3-bromopyruvate; CFA: colony formation assay; GAPDH: glyceraldehyde 3-phosphate dehydrogenase; GSH: glutathione; MCT-1: monocarboxylic acid transporter 1; NAC: N - Acetylcysteine; NEO218: perillyl alcohol conjugated to 3-bromopyruvate; POH: perillyl alcohol; ROS: reactive oxygen species; SDH: succinate dehydrogenase complex.
[0226] Summary
[0227] The anticancer agent 3-bromopyruvate (3-BP) is considered a glycolytic inhibitor that preferentially kills glycolytic cancer cells through energy depletion. However, its cytotoxic activity is dependent on drug import into cells via transmembrane monocarboxylate transporter 1 (MCT-1), which limits its anticancer potential to MCT-1-positive tumor cells. We produced and characterized an MCT-1-independent 3-BP analog, termed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com