High-affinity anti-cyclic citrullinated peptide tetravalent micro-molecule antibody and preparation method and application thereof
An anti-cyclic citrullinated peptide, small molecule antibody technology, applied in chemical instruments and methods, antibodies, antibody mimics/scaffolds, etc., can solve problems such as lack of high-affinity anti-CCP antibodies, and achieve the effect of eliminating space obstacles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: High affinity anti-cyclic citrullinated peptide single-chain variable region tetravalent small molecule antibody
[0062] This example provides a high-affinity anti-cyclic citrullinated peptide single-chain variable region tetravalent small molecule antibody based on human p53, whose sequence includes the following parts:
[0063] 1. Anti-CCP ScFv-sequence
[0064] The anti-CCP ScFv antibody gene sequence that has been constructed in the pHEN2 phage expression system in the previous research, specifically:
[0065] CAGGTGCAGCTGCAGGAGTCGGGCCCAGGAGTGGTGAAGCCTTCGGAGACCCTGTCCCTCACCTG CGCTGTCTCTGGTGGCTCCATGACTAATTATTACTGGGACTGGATTCGGCAGTCCCCAGGGAAGGGA CCGGAGTGGATTGGGTATATCTATTACAGTGGGAACACCAAGTACAACCCCTCCCTCAAGAGTCGA GTCACCATATCAGTAGACACGTCCAAGAACCAGTTCTCCCTGAGGCTGACCTCCGTGACCGCCGCA GACACGGCCGTGTATTATTGTGCGAGACATAATGCATATTACTATGACAGAAGTGGTTACTACTTCCC TGAATACTTCCGACACTGGGGCCAGGGCACCCTGGTCACCGTCTCCTCGGCATCCACCAAGGGCCC ATCGGTCACTTCGAGTGGTGGAGGCGGTAGTGCACAGGATGTTGTGAT...
Embodiment 2
[0082] Example 2: Preparation of high-affinity tetravalent small molecule antibody against cyclic citrullinated peptide single-chain variable region
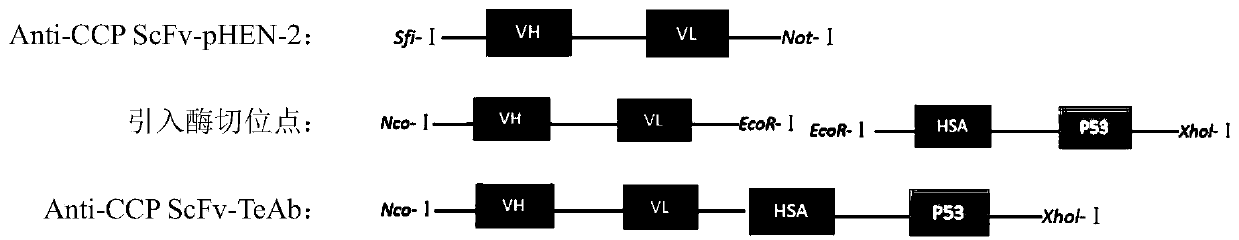
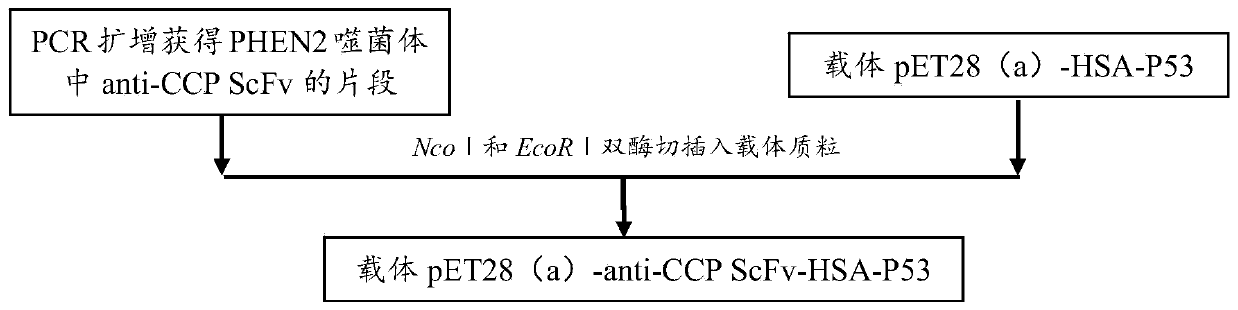
[0083] This example provides a specific preparation method for a high-affinity anti-cyclic citrullinated peptide single-chain variable region tetravalent small molecule antibody. For the construction model of pET28(a)-anti-CCP ScFv-HAS-P53, please refer to figure 1 As shown, pET28(a)-anti-CCPScFv-HAS-P53 construction technical route can refer to figure 2 Shown; The method includes the following specific steps:
[0084] 1) Extract pHEN2-anti-CCP ScFv phage plasmid DNA (Promega, A1330) and amplify it, and introduce enzyme cutting sites;
[0085] 2) digestion and linking Anti-CCP ScFv-sequence, linker protein sequence HSA-linker and P53 quadruple domain;
[0086] 3) The ligation product was transformed into competent Escherichia coli DH5a;
[0087] 4) Extract colony plasmid DNA, PCR amplification and NcoI and EcoRI double enzym...
Embodiment 3
[0112] Example 3: Detection of secretory B lymphocytes
[0113] In this embodiment, the peripheral blood mononuclear cells (PBMC) of RA patients with high active disease activity were cultured in vitro, so as to detect the effect of the anti-CCP antibody (SEQ ID NO: 2) provided in Example 1 on the secretion of CCP antibody B lymphocytes stimulated by CCP antigen. Cell number and function.
[0114] 1. Experimental materials and methods
[0115] (1) Experimental materials: 5 patients with rheumatoid arthritis who underwent arthroscopic synovectomy or surgical replacement of the joint synovium, all in line with the 1987 ACR RA classification diagnostic criteria, DAS28 score ≥ 3.2, tender joint number ≥ 8 / 68, the number of swollen joints ≥ 6 / 68, hsCRP ≥ 10mg / dl, age 35-55 years old, average 55.40±6.06 years old, all female, 5 female patients with trauma and osteoarthritis joint replacement, age 56.0±8.70 years old, Sign the informed consent form, and file with the Ethics Commit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com