Method for continuously preparing dodecyl benzene
A dodecylbenzene, high-efficiency technology, applied in the field of continuous preparation of dodecylbenzene, can solve the problems of solid acid catalyst deactivation, affecting production efficiency, easy coverage, etc., achieve rapid and uniform mixing process, and stable product quality , good quality effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
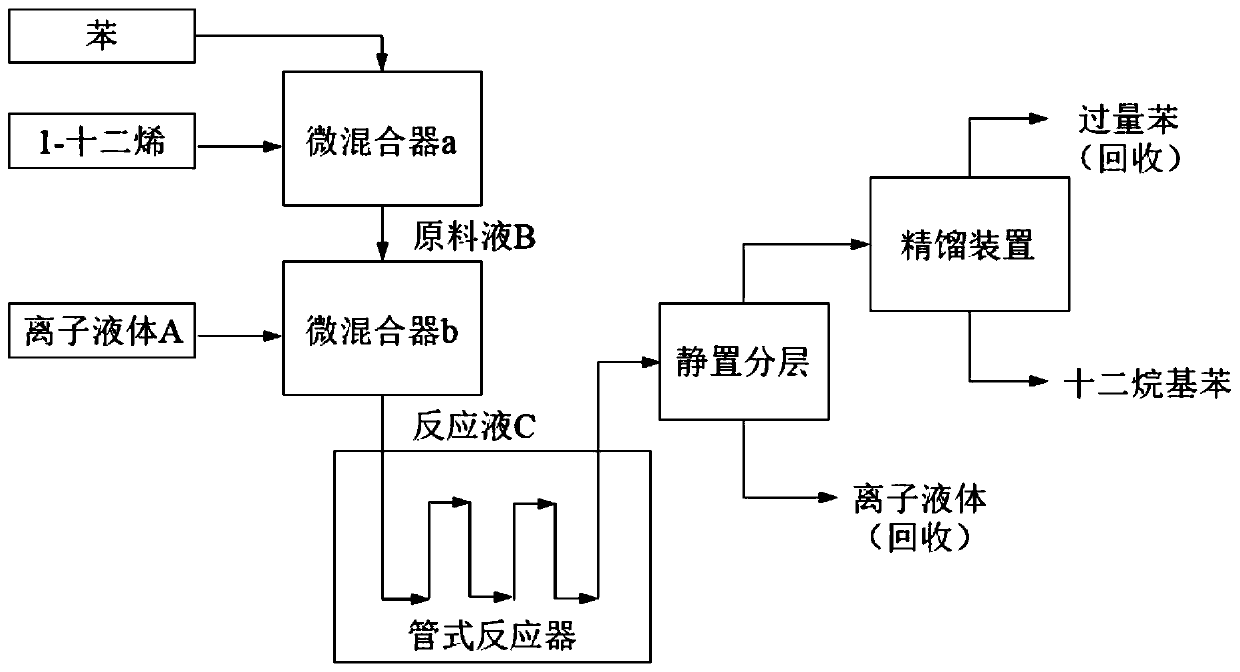
[0036] as per figure 1 The process shown is continuous preparation of dodecylbenzene:
[0037] (1) Preparation of ionic liquid A: Weigh 20.0g of trimethylamine hydrochloride in a container, heat to a constant temperature of 80°C; weigh 55.8g of aluminum chloride, and add chloride chloride in batches to trimethylamine hydrochloride while stirring. Aluminum, after adding all the aluminum chloride, continue to stir at constant temperature for 1 hour, and cool to room temperature to obtain a dark reddish brown liquid with a density of about 1.2-1.4, which is the catalyst ionic liquid A.
[0038] (2) Feed benzene and 1-dodecene into micro-mixer a at flow rates of 2 ml / min and 0.6 ml / min respectively, and mix quickly and uniformly to prepare benzene raw material liquid B.
[0039] (3) The ionic liquid A obtained in the step (1) and the raw material solution B obtained in the step (2) are continuously passed into the micro-mixer b at flow rates of 0.5 ml / min and 2.5 ml / min respectiv...
Embodiment 2
[0042] as per figure 1 The process shown is continuous preparation of dodecylbenzene:
[0043] (1) Preparation of ionic liquid A: Weigh 20.0g of trimethylamine hydrochloride in a container, heat to a constant temperature of 80°C; weigh 55.8g of aluminum chloride, and add chloride chloride in batches to trimethylamine hydrochloride while stirring. Aluminum, after adding all the aluminum chloride, continue to stir at constant temperature for 1 hour, and cool to room temperature to obtain a dark reddish brown liquid with a density of about 1.2-1.4, which is the catalyst ionic liquid A.
[0044] (2) Feed benzene and 1-dodecene into micro-mixer a at flow rates of 2 ml / min and 0.6 ml / min respectively, and mix quickly and uniformly to prepare benzene raw material liquid B.
[0045](3) The ionic liquid A obtained in the step (1) and the raw material solution B obtained in the step (2) are continuously passed into the micro-mixer b at flow rates of 0.5 ml / min and 2.5 ml / min respective...
Embodiment 3
[0048] as per figure 1 The process shown is continuous preparation of dodecylbenzene:
[0049] (1) Preparation of ionic liquid A: Weigh 20.0g of trimethylamine hydrochloride in a container, heat to a constant temperature of 80°C; weigh 55.8g of aluminum chloride, and add chloride chloride in batches to trimethylamine hydrochloride while stirring. Aluminum, after adding all the aluminum chloride, continue to stir at constant temperature for 1 hour, and cool to room temperature to obtain a dark reddish brown liquid with a density of about 1.2-1.4, which is the catalyst ionic liquid A.
[0050] (2) Feed benzene and 1-dodecene into the micro-mixer a at flow rates of 3.8ml / min and 1.2ml / min respectively, mix quickly and uniformly to prepare benzene raw material solution B.
[0051] (3) The ionic liquid A obtained in the step (1) and the raw material solution B obtained in the step (2) are continuously passed into the micro-mixer b at a flow rate of 1 ml / min and 5 ml / min respective...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com