A method for directly constructing indolo ring compounds
A technology for cyclic compounds and indole, which is applied in the field of direct synthesis of indole ring compounds through aryl side chain C-H bond activation, can solve problems such as difficult to avoid metal residues in synthetic drugs, difficult to control, high reaction selectivity of equipment, etc., to achieve production The effect of high efficiency, mild conditions and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
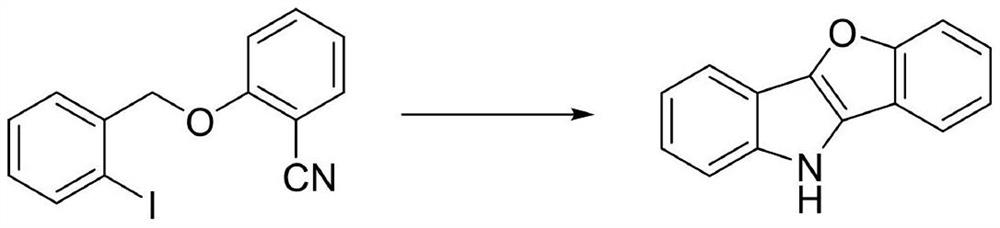
[0014] Synthesis of 10H-benzofuro[3,2-b]indole
[0015]
[0016] Add 0.5 mmol of 2-[(2-iodobenzyl)oxo]benzonitrile, 0.05 equivalents of anhydrous copper sulfate and 2.2 equivalents of potassium tert-butoxide into a 100 mL Schlenk reaction tube, dry in vacuum for 15 minutes, and Add 10mL of chlorobenzene under (or nitrogen) atmosphere, put a polytetrafluoro stopper on the reaction tube, put it in an oil bath, and react at 90°C for 12h. After the reaction was completed, the solvent was removed by filtration and concentration, separated by column chromatography, and the eluent was petroleum ether / dichloromethane / ethyl acetate (v:v:v=20:2:1), and the obtained white solid was 10H-benzene And furo[3,2-b]indole; the yield was 98%.
[0017] The white solid was analyzed as 10H-benzofuro[3,2-b]indole by chemical shift and splitting of proton nuclear magnetic resonance (1H NMR) and carbon nuclear magnetic resonance (13C NMR).
[0018] 1 H NMR (400MHz, CDCl 3 )δ8.01(s,1H),7.83(d,J=...
Embodiment 2
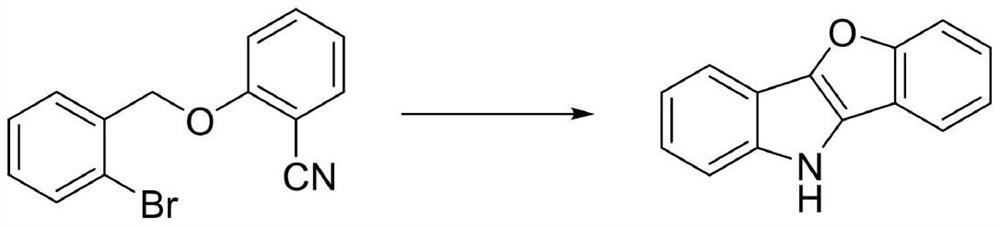
[0020] Synthesis of 10H-benzofuro[3,2-b]indole
[0021]
[0022] Add 0.5mmol of 2-[(2-bromobenzyl)oxo]benzonitrile, 0.05 equivalent of anhydrous copper sulfate and 2.2 equivalents of potassium tert-butoxide into a 100mL Schlenk reaction tube, dry in vacuum for 15 minutes, and Add 10mL of chlorobenzene under (or nitrogen) atmosphere, put a polytetrafluoro stopper on the reaction tube, put it in an oil bath, and react at 90°C for 12h. After the reaction was completed, the solvent was removed by filtration and concentration, separated by column chromatography, and the eluent was petroleum ether / dichloromethane / ethyl acetate (v:v:v=20:2:1), and the obtained white solid was 10H-benzene Andfuro[3,2-b]indole; the yield was 77%.
[0023] The white solid was analyzed as 10H-benzofuro[3,2-b]indole by chemical shift and splitting of proton nuclear magnetic resonance (1H NMR) and carbon nuclear magnetic resonance (13C NMR).
[0024] 1 H NMR (400MHz, CDCl 3 )δ8.01(s,1H),7.83(d,J=7.6...
Embodiment 3
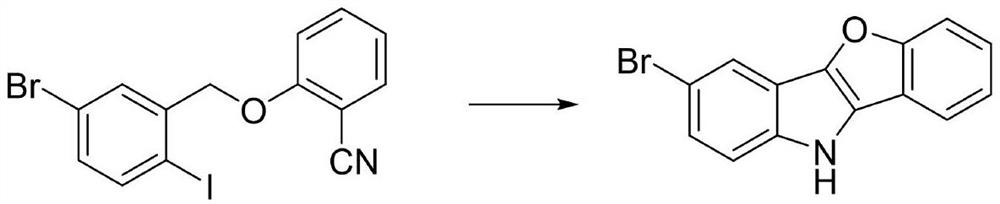
[0026] Synthesis of 3-bromo-10H-benzofuro[3,2-b]indole
[0027]
[0028] Add 0.5 mmol of 2-[(5-bromo-2-iodobenzyl) oxo]benzonitrile, 0.05 equivalents of anhydrous copper sulfate and 2.2 equivalents of potassium tert-butoxide into a 100 mL Schlenk reaction tube, and dry in vacuum for 15 minutes , add 10 mL of chlorobenzene under an argon (or nitrogen) atmosphere, add a polytetrafluoro stopper to the reaction tube, put it in an oil bath, and react at 90° C. for 15 h. After the reaction was completed, the solvent was removed by filtration and concentration, separated by column chromatography, the eluent was petroleum ether / dichloromethane / ethyl acetate (v:v:v=20:2:1), and the obtained white solid was 3-bromo -10H-benzofuro[3,2-b]indole; 83% yield.
[0029] The white solid was analyzed to be 3-bromo-10H-benzofuro[3,2-b]indole by chemical shift and fragmentation of proton nuclear magnetic resonance (1H NMR) and carbon nuclear magnetic resonance (13C NMR).
[0030] 1 H NMR (40...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com