Recombinant human fallicle-stimulating hormone and preparation method thereof
A human follicle-stimulating hormone and follicle-stimulating hormone technology, applied in the protein field, can solve the problems of high cost of recombinant FSH products and unaffordable economic burden of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0113] The invention provides a method for preparing recombinant follicle-stimulating hormone, comprising the following steps:
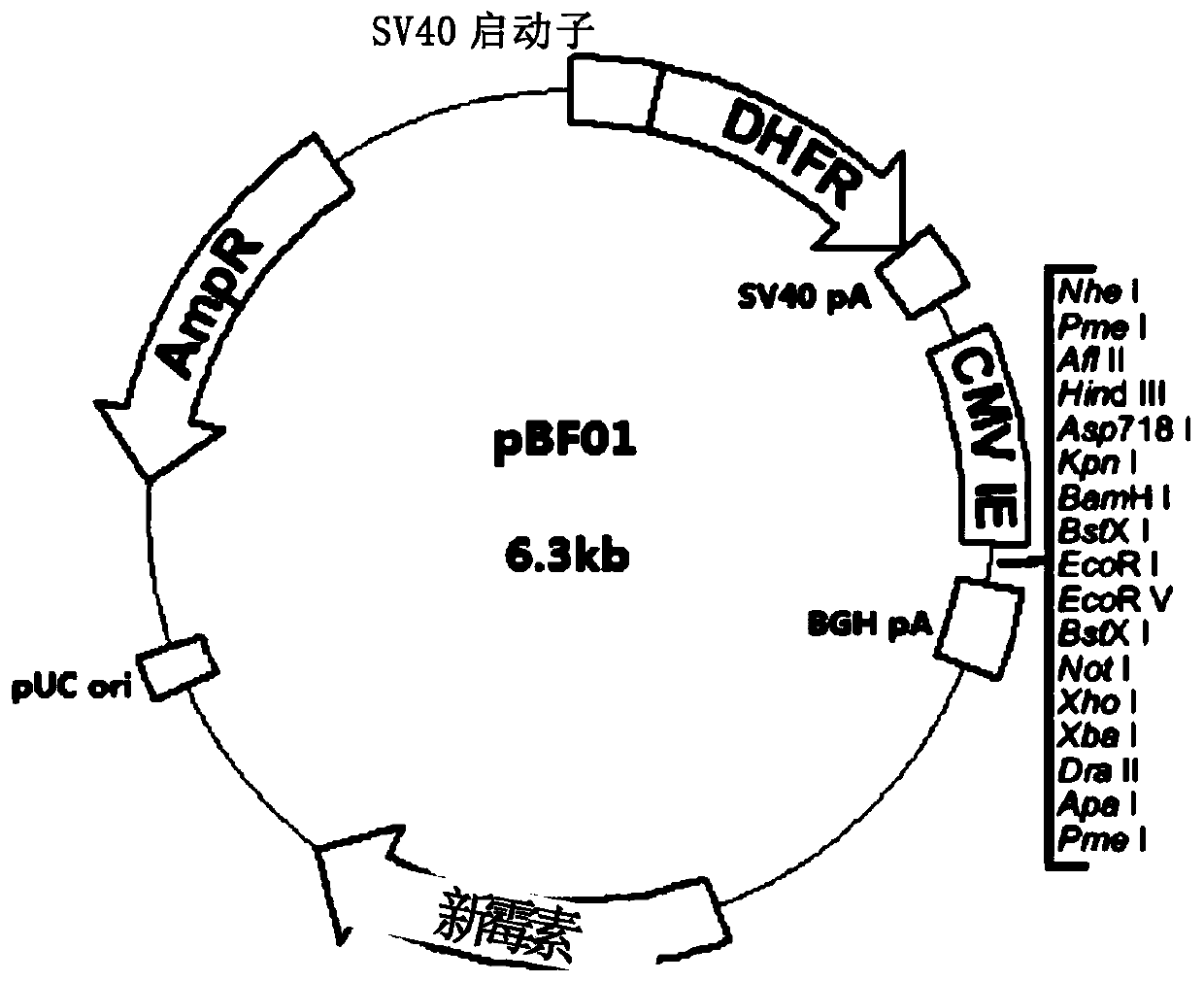
[0114] (1) Construct the eukaryotic expression vector PGM that can express FSHα subunit gene and FSHβ subunit gene simultaneously, and contain DHFR expression unit and KTPA-CMV.
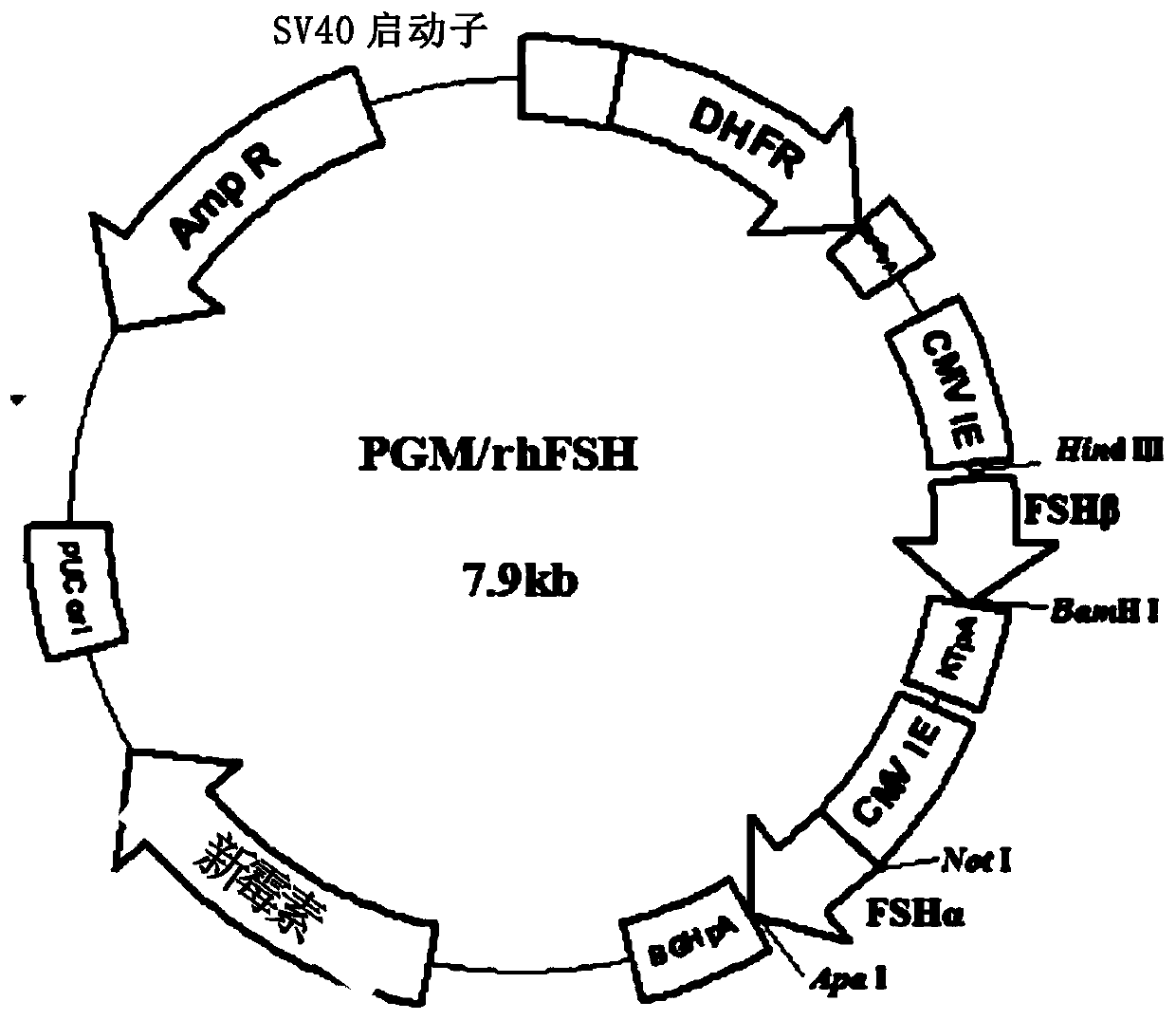
[0115] (2) The FSHα subunit gene and the FSHβ subunit gene are operatively inserted into PGM to obtain the transfection vector PGM / rhFSH capable of simultaneously expressing the FSHα subunit protein and the FSHβ subunit protein, wherein the FSHα subunit gene contains The cDNA sequence and the upstream intron sequence of the subunit protein, the FSHβ subunit gene includes the cDNA sequence and the upstream intron sequence encoding the FSHβ subunit protein;
[0116] (3) transfecting the transfection vector into eukaryotic cells and culturing; and
[0117] (4) The culture supernatant was successively subjected to ultrafiltration, ammonium sulfate precipitation, hydrophobic ch...
Embodiment 1
[0144] Example 1: Human FSHα subunit gene and FSHβ subunit gene sequence cloning
[0145] The inventors found that the FSH α subunit gene can express the final FSH α subunit, but the expression level of the FSH α subunit is not high. Based on experience, 8 FSH α subunit genes were designed by introducing enzyme cleavage sites, KOZAK sequences and signal peptides The sequence was used for expression research, including SEQ ID NO: 1. After research, it was found that the expression levels of the designed 8 FSHα subunit sequences were different, some were high, and some were low. Among them, the FSHα subunit gene SEQ ID NO: 1 has the highest expression level, so SEQ ID NO:1 was selected for cloning.
[0146] In addition, FSHβ subunit gene can express the final FSHβ subunit, but the expression level of FSHβ subunit is not high. Expression research, including SEQ ID NO: 2, after research, it was found that the expression levels of the designed 10 FSHβ subunit sequences varied, som...
Embodiment 2
[0148] Embodiment 2: the generation of PGM
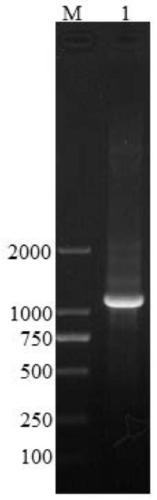
[0149] The dhfr gene expression unit on the pSV2-dhfr vector (ATCC 37146) was cloned into the pcDNA3.1(+) vector (Invitrogen). The inventors designed and introduced the Blg II and Mlu I restriction sites, screened to obtain the sequence comprising SEQ ID NO: 5 and SEQ ID NO6, and performed routine PCR on the dhfr gene expression unit on the pSV2-dhfr vector (ATCC 37146) clone. The cloning product was confirmed by electrophoresis on 1% agarose gel (containing EB), and its length was 1.1kb, which was consistent with the prediction (see figure 1 ). The dhfr gene expression unit includes SV40 early promoter, dhfr coding region, SV40 termination and polyA signal sequence.
[0150] Table 1 - Oligonucleotides
[0151]
[0152]
[0153] The cloned dhfr gene expression unit was double-digested (Blg II and MluI) using a conventional enzyme digestion system, and the fragments were recovered with a gel recovery kit. Carry out double ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com