A kind of preparation method and application of highly active tetranuclear polymer
A copper compound, mononuclear technology, applied in the application field of anti-diabetic drugs, can solve problems such as no reports on research, and achieve the effects of simple preparation method, low cost and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
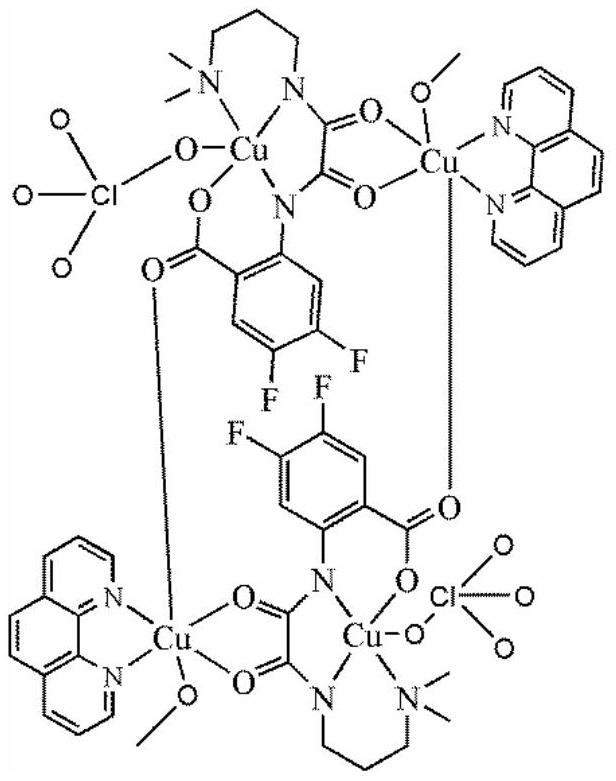
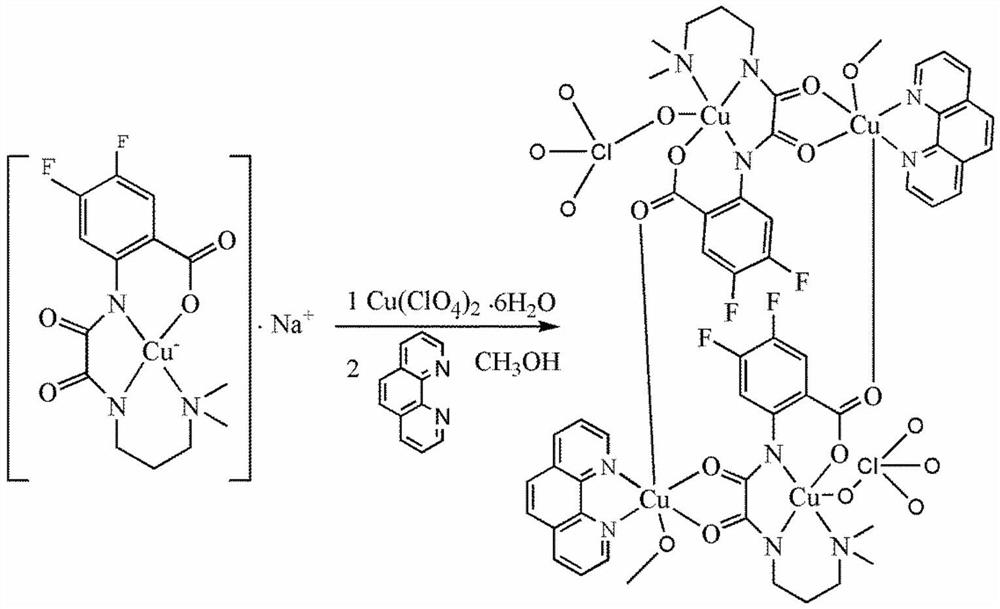
[0017] Dissolve 2 mmol of oxamide mononuclear copper ligand in 10 ml of methanol, dissolve 2 mmol of copper perchlorate in 5 ml of methanol, add it to the methanol solution of the above mononuclear copper ligand, react at 60 ° C for 1 hour, and then dissolve the dissolved Add 2 mmol of 1,10-phenanthroline ligand methanol solution to the mixture of the above ligands, reflux at 60°C for 5 hours, filter, and the filtrate slowly volatilizes for 1 week to obtain blue blocky crystals, which are difluorinated Amide Ligand Tetranuclear Copper Compounds.
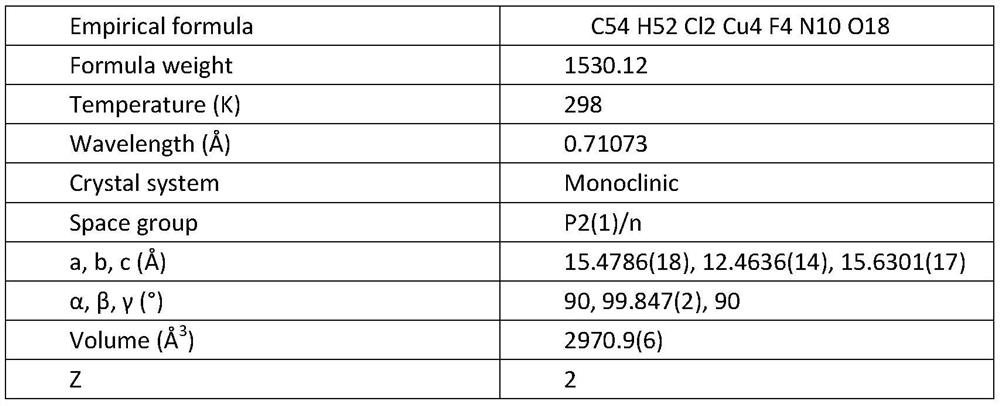
[0018] Infrared, elemental analysis and X-ray single crystal diffraction results:
[0019] Infrared spectrum (KBr, cm -1 ):ν(N-H)3419νas(C=O)16281574;ν s (C6H6-H) 1464 726
[0020] Elemental analysis: Calculated: C, 42.39; H, 3.43; N, 9.15%. Found: C, 42.37; H, 3.45; N, 9.16%.
[0021] X-ray single crystal diffraction results:
[0022]
[0023] Molecular structural formula of tetranuclear copper compound containing difluoroox...
Embodiment 2
[0028] Dissolve 2 mmol of the oxamide mononuclear copper ligand in 20 ml of methanol, dissolve 2 mmol of copper chloride in 10 ml of methanol, add it to the methanol solution of the above mononuclear copper ligand, react at 60 ° C for 1 hour, and then dissolve 2 mmol The methanol solution of 1,10-phenanthroline ligand was added to the mixture of the above ligands, refluxed at 60°C for 5 hours, filtered, and the filtrate was slowly volatilized for 1 week to obtain blue blocky crystals. It is tetranuclear copper compound containing difluorooxamide ligand.
Embodiment 3
[0030] Dissolve 2 mmol of oxamide mononuclear copper ligand in 10 ml of methanol, dissolve 2 mmol of copper bromide in 5 ml of methanol and add it to the methanol solution of the above mononuclear copper ligand, react at 60 ° C for 1 hour, and then dissolve 2 mmol The methanol solution of 1,10-phenanthroline ligand was added to the mixture of the above ligands, refluxed at 60°C for 5 hours, filtered, and the filtrate was slowly volatilized for 1 week to obtain blue blocky crystals. It is tetranuclear copper compound containing difluorooxamide ligand.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com