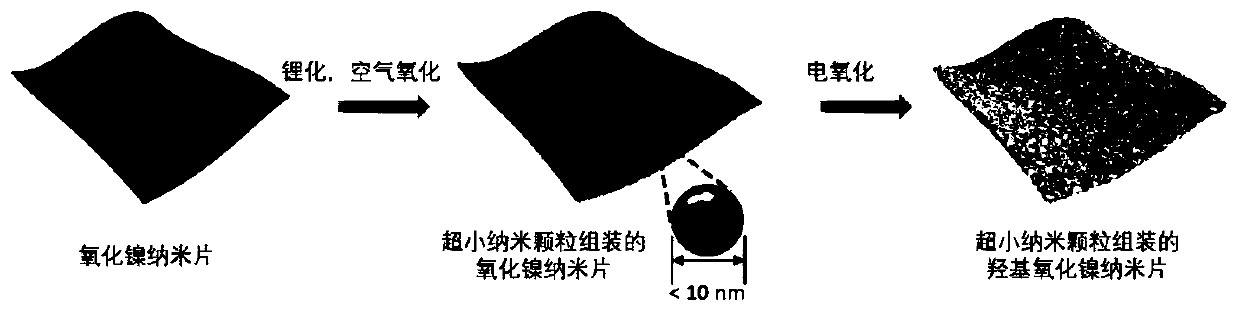
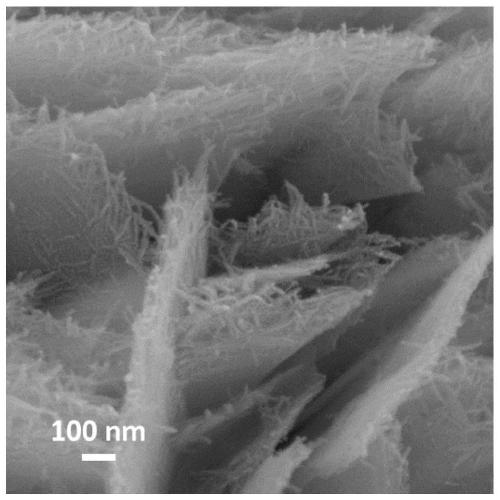
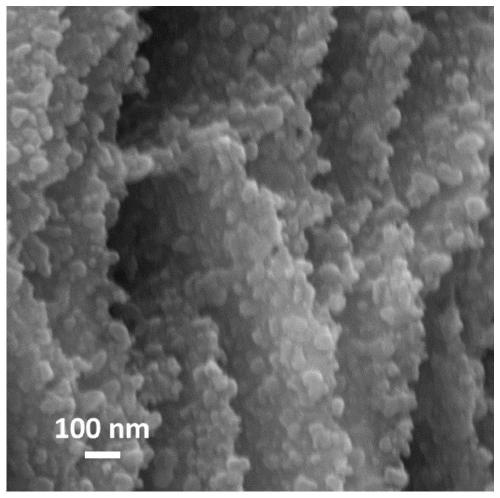
Low-crystallization graded nickel oxyhydroxide nano-sheet array and preparation method and application thereof
A technology of nickel oxyhydroxide and nanosheet arrays, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, chemical/physical processes, etc. Contact and other issues to achieve the effect of increasing quantity, high quality specific activity catalytic performance, and improving mass specific activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The preparation method of the low-crystalline graded nickel oxyhydroxide nanosheet array includes:
[0037] 1) Weigh 3mmol Ni(NO 3 ) 2 ·6H 2 O, 2 mmol NH 4 F and 5mmol CO(NH 2 ) 2 Dissolve in 35mL deionized water, stir to obtain a clear and transparent mixed solution;
[0038] 2) Transfer the mixed solution obtained in step 1) to a reaction kettle, and add commercial metal nickel foam with a size of 2cm*4cm. After hydrothermal reaction at 120°C for 6 hours, take out the nickel foam sample after natural cooling, and use alcohol and After washing with water and drying, the nickel hydroxide nanosheet array precursor was obtained;
[0039] 3) Calcining the nickel hydroxide nanosheet array precursor obtained in step 2) in a muffle furnace at 400° C. for 3 hours to obtain a nickel oxide nanosheet array grown on nickel foam;
[0040] 4) Cut the nickel foam sample obtained in step 3) into discs with a diameter of 1 cm; assemble a lithium-ion half-cell, fully discharge to...
Embodiment 2
[0045] The preparation method of the low-crystalline graded nickel oxyhydroxide nanosheet array includes:
[0046] 1) Weigh 2.5mmol Ni(NO 3 ) 2 ·6H 2 O, 2 mmol NH 4 F and 5mmol CO(NH 2 ) 2 Dissolve in 35mL deionized water, stir to obtain a clear and transparent mixed solution;
[0047] 2) Transfer the mixed solution obtained in step 1) to a reaction kettle, and add commercial metal nickel foam with a size of 2cm*4cm. After hydrothermal reaction at 120°C for 6 hours, take out the nickel foam sample after natural cooling, and use alcohol and After washing with water and drying, the nickel hydroxide nanosheet array precursor was obtained;
[0048] 3) Calcining the nickel hydroxide nanosheet array precursor obtained in step 2) in a muffle furnace at 400° C. for 3 hours to obtain a nickel oxide nanosheet array grown on metallic nickel foam;
[0049] 4) Cut the nickel foam sample obtained in step 3) into discs with a diameter of 1 cm; assemble a lithium-ion half-cell, fully d...
Embodiment 3
[0053] The preparation method of the low-crystalline graded nickel oxyhydroxide nanosheet array includes:
[0054] 1) Weigh 3mmol Ni(NO 3 ) 2 ·6H 2 O, 2 mmol NH 4 F and 5mmol CO(NH 2 ) 2 Dissolve in 35mL deionized water, stir to obtain a clear and transparent mixed solution;
[0055] 2) Transfer the mixed solution obtained in step 1) to a reaction kettle, and add a commercial metallic nickel foam with a size of 3cm*3cm. After hydrothermal reaction at 120°C for 6 hours, take out the nickel foam sample after natural cooling, and wash with alcohol and After washing with water and drying, the nickel hydroxide nanosheet array precursor was obtained;
[0056] 3) Calcining the nickel hydroxide nanosheet array precursor obtained in step 2) in a muffle furnace at 400° C. for 3 hours to obtain a nickel oxide nanosheet array grown on metallic nickel foam;
[0057] 4) Cut the nickel foam sample obtained in step 3) into discs with a diameter of 1 cm; assemble a lithium-ion half-cell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com