A kind of brassinosterol derivatization reagent and its preparation method and application
A technology of brassinosterol and derivatization reagents, applied in the field of organic chemistry and analytical chemistry, can solve the problems of easy decomposition, unstable derivatized products, poor sensitivity, etc., and achieve the effect of not easy to decompose, stable decomposition, and improve sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of 2-methyl-4-phenylaminomethylbenzeneboronic acid
[0023] Add 4-formyl-2-methylbenzeneboronic acid (0.92mmol), aniline (0.92mmol), acetic acid (0.01mmol) and methanol (25mL) into the reaction flask, stir the reaction at room temperature for 0.5 hours, and then add cyano Sodium borohydride (0.92mmol) reduced the carbon-nitrogen double bond, continued the reaction for 10 hours, then ended the reaction, evaporated the methanol solvent, added 30mL saturated saline and 30mL ethyl acetate to dissolve the solid, collected the organic layer, added anhydrous sodium sulfate to dry, The product was purified by silica gel column chromatography to obtain 2-methyl-4-phenylaminomethylbenzeneboronic acid in a yield of 44.0%. Product Characterization Data 1 H NMR (400MHz, DMSO-d 6 )δ7.94(s, 2H), 7.36(d, J=7.4Hz, 1H), 7.10(d, J=8.1Hz, 2H), 7.01(t, J=9.0Hz, 2H), 6.53(dd, J=8.6,1.0Hz,2H),6.47(m,1H),6.22(m,1H),4.19(d,J=6.1Hz,2H),2.37(s,3H).m / z(M+H ) + = 24...
Embodiment 2
[0024] Embodiment 2: compare with reported derivatization reagent sensitivity
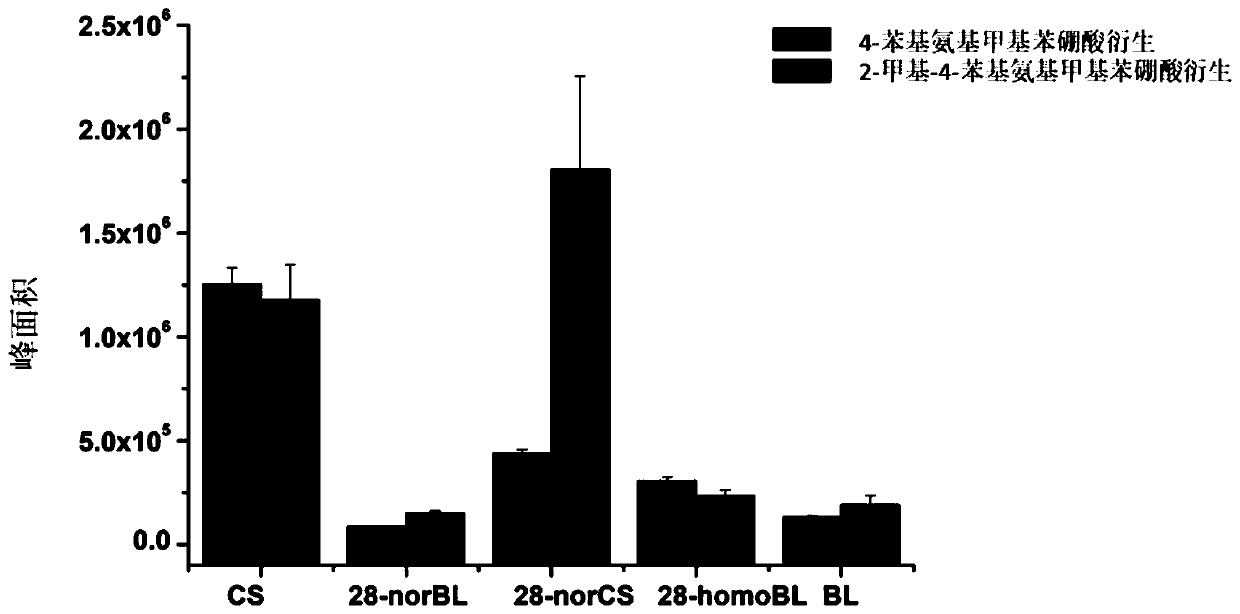
[0025] The post-derivatization sensitivity of the reported brassinosterol derivatization reagent 4-phenylaminomethylphenylboronic acid was compared. Mix 100μL brassinosterol mixed standard solution (including: CS, 10ng / mL; 28-norBL, 10ng / mL; 28-norCS, 10ng / mL; 28-homoBL, 10ng / mL; BL, 10ng / mL) with 100μL 4-Phenylaminomethylphenylboronic acid (4mM) and 2-methyl-4-phenylaminomethylphenylboronic acid (4mM) were reacted at room temperature for 10 minutes, and then 5 μL of the reaction solution was injected into the ultra-high liquid chromatography- Electrospray-triple quadrupole tandem mass spectrometry was used for analysis.
[0026] Such as figure 1 Shown; The mass spectrum response of the brassinosterol compound (28-norBL; 28-norCS) without methyl or ethyl functional group at the C24 position is good after derivatization with 2-methyl-4-phenylaminomethylbenzeneboronic acid Based on the reported de...
Embodiment 3
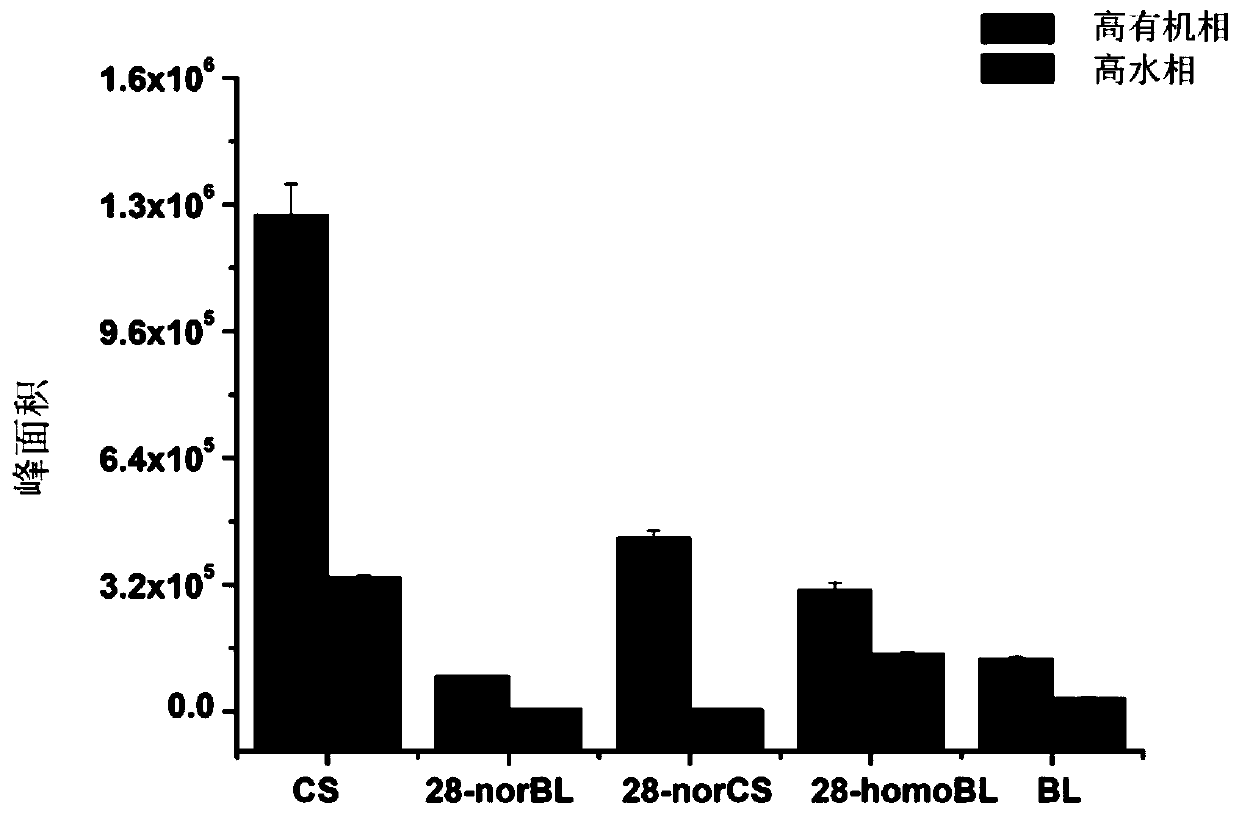
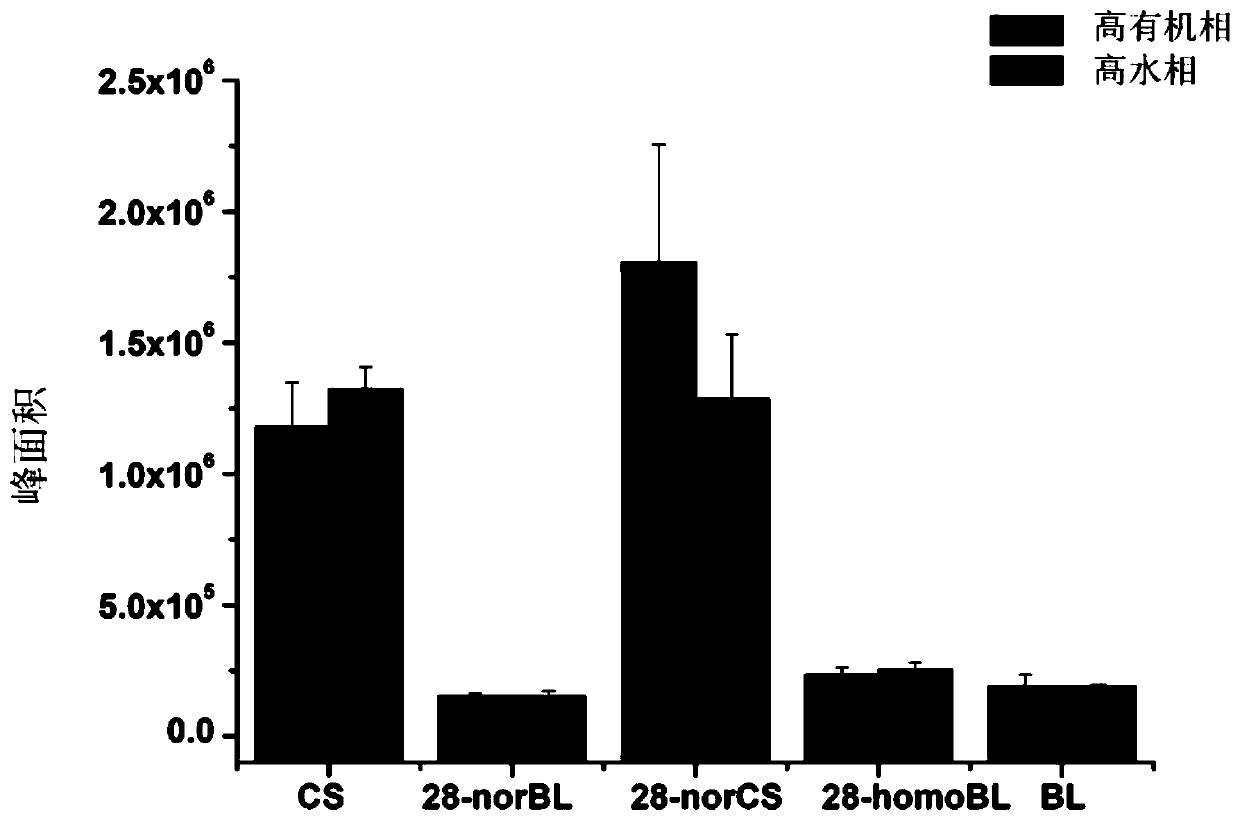
[0027] Embodiment 3: Derivative Stability Investigation
[0028] The post-derivatization stability of the reported brassinosterol derivatization reagent 4-phenylaminomethylphenylboronic acid was compared. Mix 100μL brassinosterol mixed standard solution (including: CS, 10ng / mL; 28-norBL, 10ng / mL; 28-norCS, 10ng / mL; 28-homoBL, 10ng / mL; BL, 10ng / mL) with 100μL 4-Phenylaminomethylphenylboronic acid (4mM) and 2-methyl-4-phenylaminomethylphenylboronic acid (4mM) were reacted for 10 minutes, and then 5 μL of the reaction solution was injected into ultra-high liquid chromatography-electrospray- Triple quadrupole tandem mass spectrometry was used for analysis. The separation chromatographic column used was Waters C18 column (2.1×100mm, 1.8 μm), and the mobile phase was A: water phase, B: acetonitrile. Derivatives were separated using high organic and high aqueous gradients, respectively. High organic gradient: 0-5 minutes: 70% vol B, 5-10 minutes: 90% vol B, 10-12 minutes: 70% vol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com