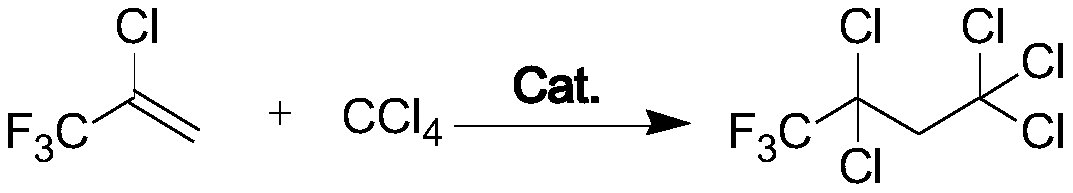
Preparation method of 1,1,1,3,3-pentachloro-4,4,4-trifluorobutane
A technology for trifluorobutane and trifluoropropene, which is applied in the field of preparation of hydrofluorochloroalkanes, can solve the problems of large amount of metal, waste, loss of trifluoropropene, etc., and achieves high industrial application prospects and the effect of improving catalytic efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 2.14 grams of ferric chloride, 13.16 grams of tributyl phosphate and 50 grams of carbon tetrachloride solution in the 1.5L stainless steel belt stirred autoclave, add 4.39 grams of iron powder and 450 grams of carbon tetrachloride at the solid feed port, pre- After 26 grams of 2-chloro-3,3,3-trifluoropropene was introduced, the temperature was raised to 115° C., and 2-chloro-3,3,3-trifluoropropene was continuously introduced into the gas phase until 2-chloro-3 , After the amount of 3,3-trifluoropropene feeding reaches 100 grams, stop feeding, continue to stir the reaction to a pressure of 0.3 MPa, cool down to stop the reaction, take a sample for GC analysis, and obtain the product after vacuum distillation.
[0018] The product is detected by NMR, and the data are as follows:
[0019] 1 H NMR (500MHz, CDCl 3 )δ3.72(s,2H);
[0020] 13 C NMR (500MHz, CDCl 3 )δ121.8(q, J=281.3Hz), 92.6, 79.7(q, J=34.8Hz), 59.1;
[0021] 19 F NMR (470MHz, CDCl 3 )δ-80.2(s, CF ...
Embodiment 2~6
[0024] Examples 2-6 Prepare 1,1,1,3,3-pentachloro-4,4,4-trifluorobutane according to the same preparation method in Example 1, the difference is that the reaction four in Example 1 The ratio of chlorinated carbon: 2-chloro-3,3,3-trifluoropropene is 2.5:1, while the telomerization ratios in Examples 2-6 are 1:1, 2:1, 5:1, 7:1 respectively , 10:1. The reaction results of Examples 2-6 are shown in Table 1.
[0025] Table 1 Screening of telomerization ratio
[0026] Example The molar ratio of Conversion rate(%) selectivity (%) 2 1:1 90.5 84.1 3 2:1 93.3 96.1 4 5:1 94.5 95.0 5 7:1 95.6 94.3 6 10:1 96.8 96.2
Embodiment 7~10
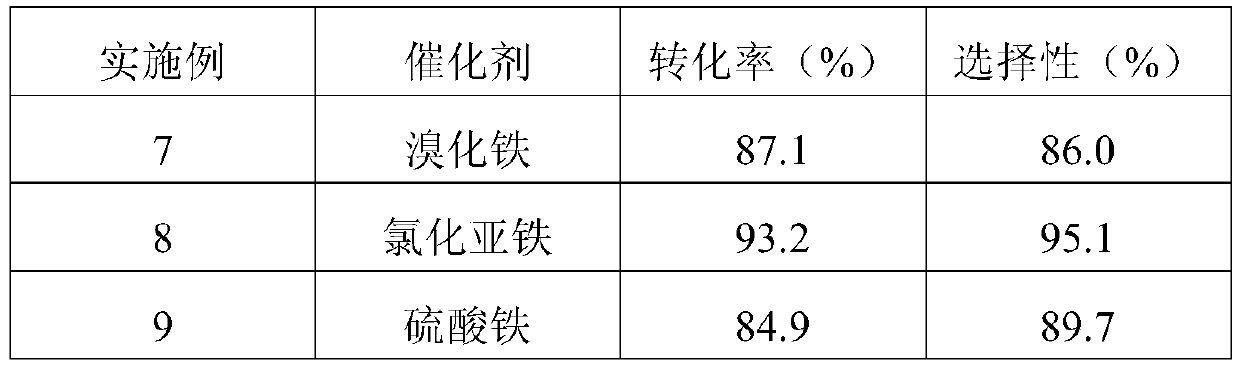
[0028] Examples 7-10 Prepare 1,1,1,3,3-pentachloro-4,4,4-trifluorobutane according to the same preparation method in Example 1, the difference is the reaction catalyst in Example 1 It is ferric chloride, and it is respectively ferric bromide, ferrous chloride, ferric sulfate or ferric acetylacetonate among the embodiments 7-10. The reaction results of Examples 7-10 are shown in Table 2.
[0029] Table 2 Catalyst screening
[0030]
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com