Synthesis method of 1-(4-aminopyridine-2-yl)ethanone
A synthesis method and aminopyridine technology, applied in the direction of organic chemistry, etc., can solve the problems of harsh equipment drying and reaction temperature control, poor economic benefit and environmental impact, and complicated post-processing process, and achieve easy operation of post-processing and purification. , risk reduction, mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
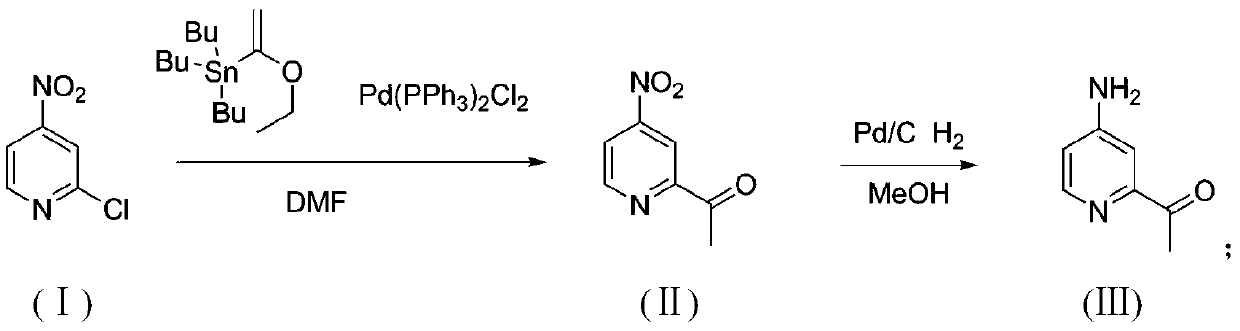
[0027] ① Mix 25g (1eq) 2-chloro-4-nitropyridine, 65g (1.15eq) tributyl (1-ethoxyethylene) tin and 3g (0.03eq) Pd (PPh 3 ) 2 Cl 2 Add 300ml of DMF, under argon protection, react at 85°C for 3h, pour 1L of ice water into it at room temperature, extract with EA, spin dry, add 300ml of 2M hydrochloric acid aqueous solution, stir the whole system at room temperature overnight, extract with EA, mix the sample and pass through the column to purify the compound II 26g, yield 99%.
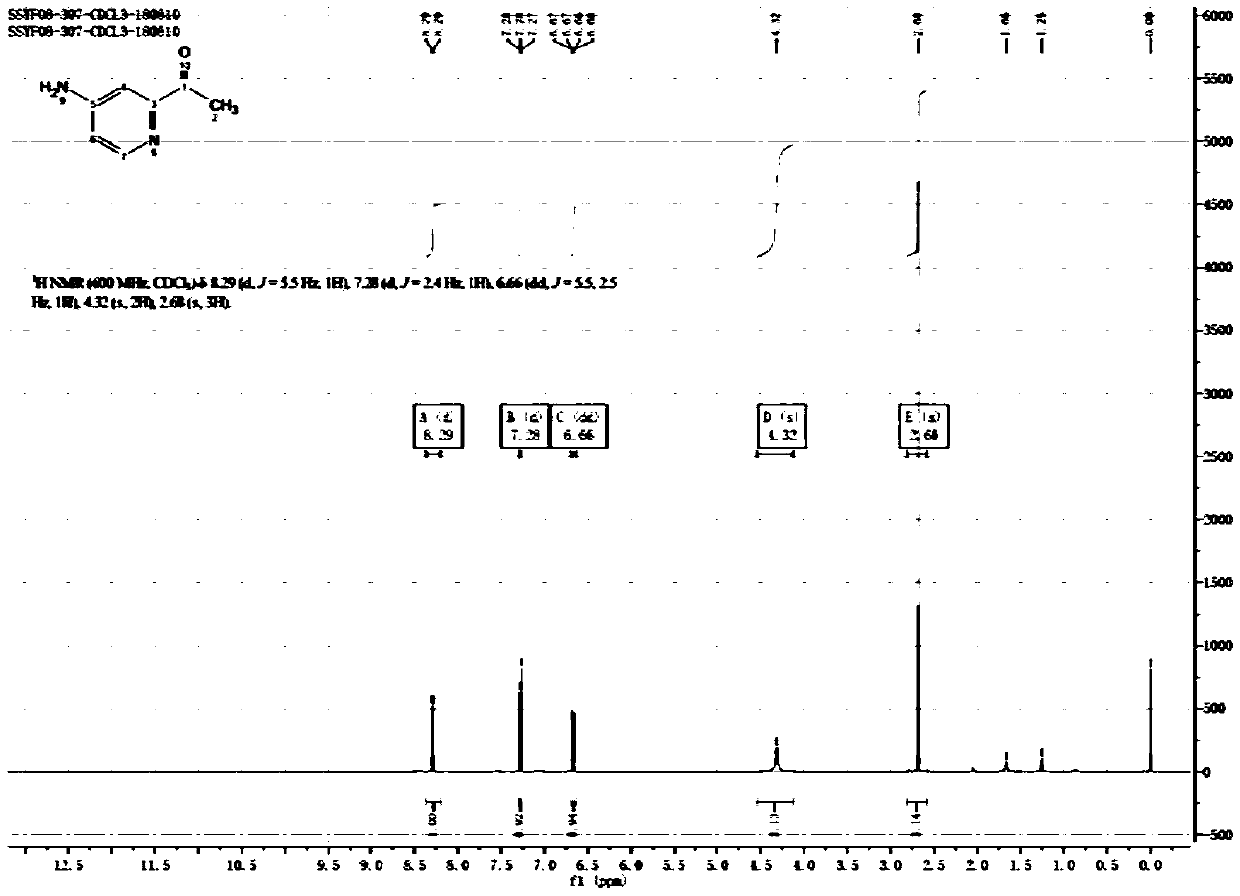
[0028] ②Add 26g of compound II to 200ml of methanol, add 2g of Pd / C, and react overnight with hydrogen gas; TLC, the reaction is complete, spin dry and filter with PE beating to obtain 19g of the target product 1-(4-aminopyridin-2-yl)ethanone , yield 89.5%, H NMR spectrum such as figure 1 shown.
Embodiment 2
[0030]
[0031] ① Mix 5g (1eq) 2-chloro-4-nitropyridine, 12.5g (1.1eq) tributyl (1-ethoxyethylene) tin and 0.7g (0.03eq) Pd (PPh 3 ) 2 Cl 2 Add 50ml of NMP, under argon protection, react at 90°C for 3h, drop to room temperature and pour 200ml of ice water, extract with EA, spin dry, add 50ml of 2M hydrochloric acid aqueous solution, stir overnight at room temperature, extract with EA, mix the sample and pass through the column to obtain 23g of compound , yield 58%.
[0032] ②Add 3g of compound 2 to 30ml of methanol, add 0.3g of Pd / C, and react overnight with hydrogen gas, TLC, the reaction is complete, spin dry, and beat with PE to obtain 2.1g of 1-(4-aminopyridin-2-yl)ethanone, Yield 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com