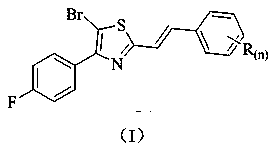
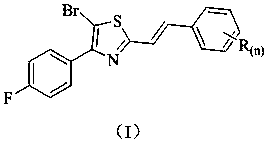
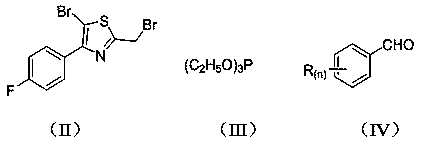Stilbene analogue containing thiazole ring structure and synthesis method and application thereof
A synthesis method and a technology containing a thiazole ring can be applied in the fields of drug combination, resistance to vector-borne diseases, organic chemistry, etc., and can solve the problems of structure and biological activity that have not been reported in literature, and achieve the effect of simple preparation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Synthesis of Compound Ia (R(n)=H):
[0028]Add 5-bromo-2-(bromomethyl)-4-(4-fluorophenyl)thiazole (3.7 g, 10 mmol) and triethyl phosphite (2.5 g, 15 mmol) into a 100 mL three-necked flask, and heat to The reaction was carried out at 100°C, and the progress of the reaction was detected by TLC, and the reaction was completed after about 3 hours. Concentrate and remove excess triethyl phosphite to obtain a concentrated solution; add DMF (2.9 g, 40 mmol), benzaldehyde (1.1 g, 10 mmol) and sodium hydroxide (0.4 g, 10 mmol) to the resulting concentrated solution React at room temperature. The reaction process was detected by TLC, and the reaction was completed in about 4 hours. Then, the reaction solution was poured into ice water (120 mL), stirred, and a solid was precipitated, filtered, and the filter cake was recrystallized with ethyl acetate to obtain 1.9 g of a yellow solid, which was (E) -5-bromo-4-(4-fluorophenyl)-2-styrylthiazole, the calculated yield is 5...
Embodiment 2
[0031] The synthesis of embodiment 2 compound Ib (R (n)=o-chloro):
[0032] Add 5-bromo-2-(bromomethyl)-4-(4-fluorophenyl)thiazole (3.7 g, 10 mmol) and triethyl phosphite (3.3 g, 20 mmol) into a 100 mL three-necked flask, heat The reaction was carried out at 120°C, and the progress of the reaction was detected by TLC, and the reaction was completed after about 1.5 h. Concentrate and remove excess triethyl phosphite to obtain a concentrated solution; add DMF (4.0 g, 55 mmol), o-chlorobenzaldehyde (1.4 g, 10 mmol) and sodium hydroxide (1.3 g, 32 mmol ) react at room temperature. The reaction process was detected by TLC, and the reaction was completed in about 4.5 hours. Then, the reaction solution was poured into ice water (130 mL), stirred, and a solid precipitated, filtered, and the filter cake was recrystallized with n-hexane to obtain 1.86 g of a yellow solid, which was (E) -2-(2-chlorostyryl)-5-bromo-4-(4-fluorophenyl)thiazole, the calculated yield is 47.3%. m.p.: 124~12...
Embodiment 3
[0035] Example 3 Synthesis of Compound Ic (R(n)=p-chloro):
[0036] Add 5-bromo-2-(bromomethyl)-4-(4-fluorophenyl)thiazole (3.7 g, 10 mmol) and triethyl phosphite (2.8 g, 17 mmol) into a 100 mL three-necked flask, and heat to The reaction was carried out at 130°C, and the progress of the reaction was detected by TLC, and the reaction was completed after about 1 h. Concentrate and remove excess triethyl phosphite to obtain a concentrated solution; add DMF (3.7g, 50mmol), p-chlorobenzaldehyde (1.7g, 12 mmol) and sodium hydroxide (0.9 g, 22 mmol) to the resulting concentrated solution ) react at room temperature. The reaction process was detected by TLC, and the reaction was completed in about 4 hours. Then, the reaction solution was poured into ice water (185 mL), stirred, and a solid precipitated, filtered, and the filter cake was recrystallized with n-hexane to obtain 2.4 g of a yellow solid, namely (E)- 2-(4-chlorostyryl)-5-bromo-4-(4-fluorophenyl)thiazole, the calculated y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com