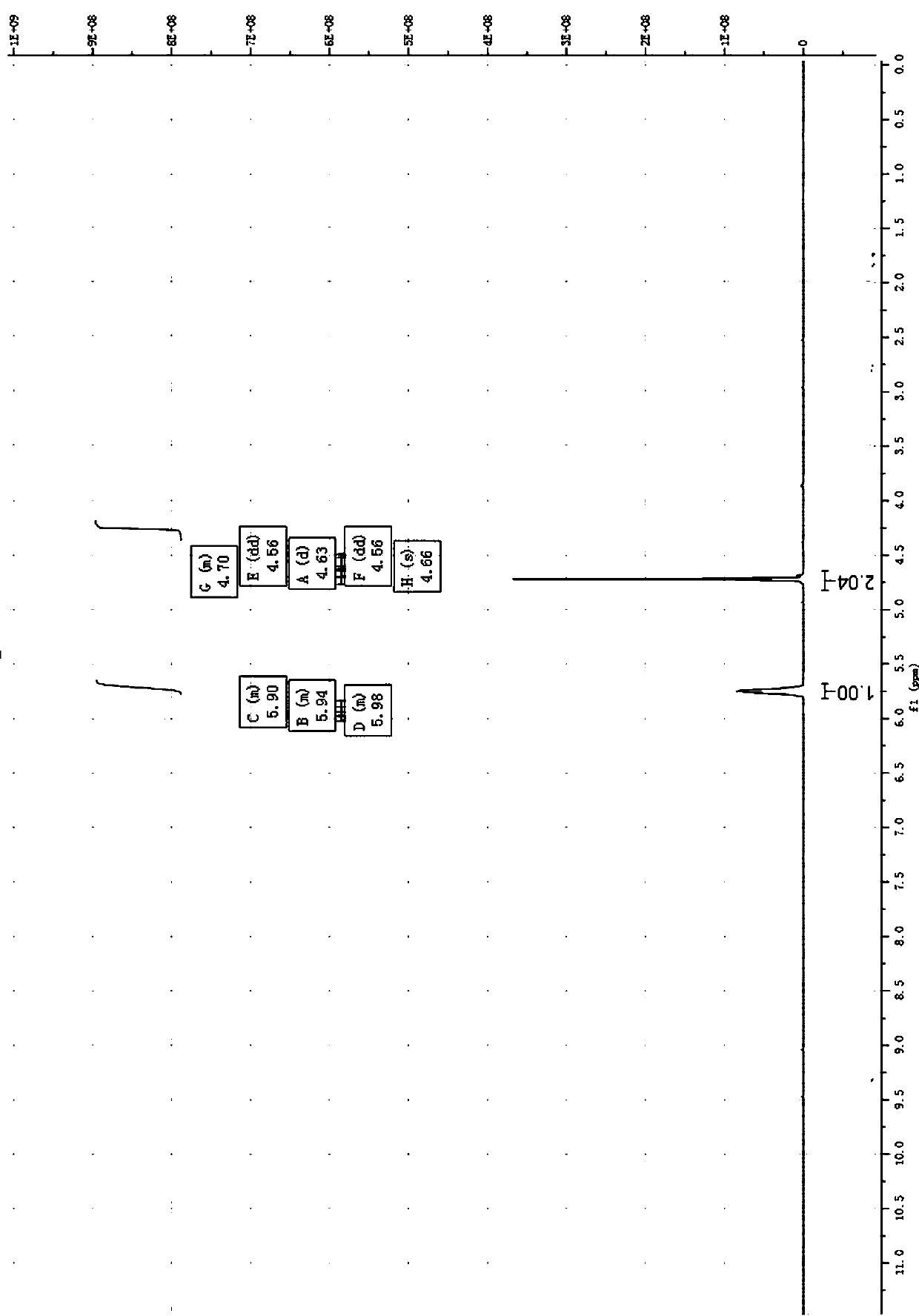
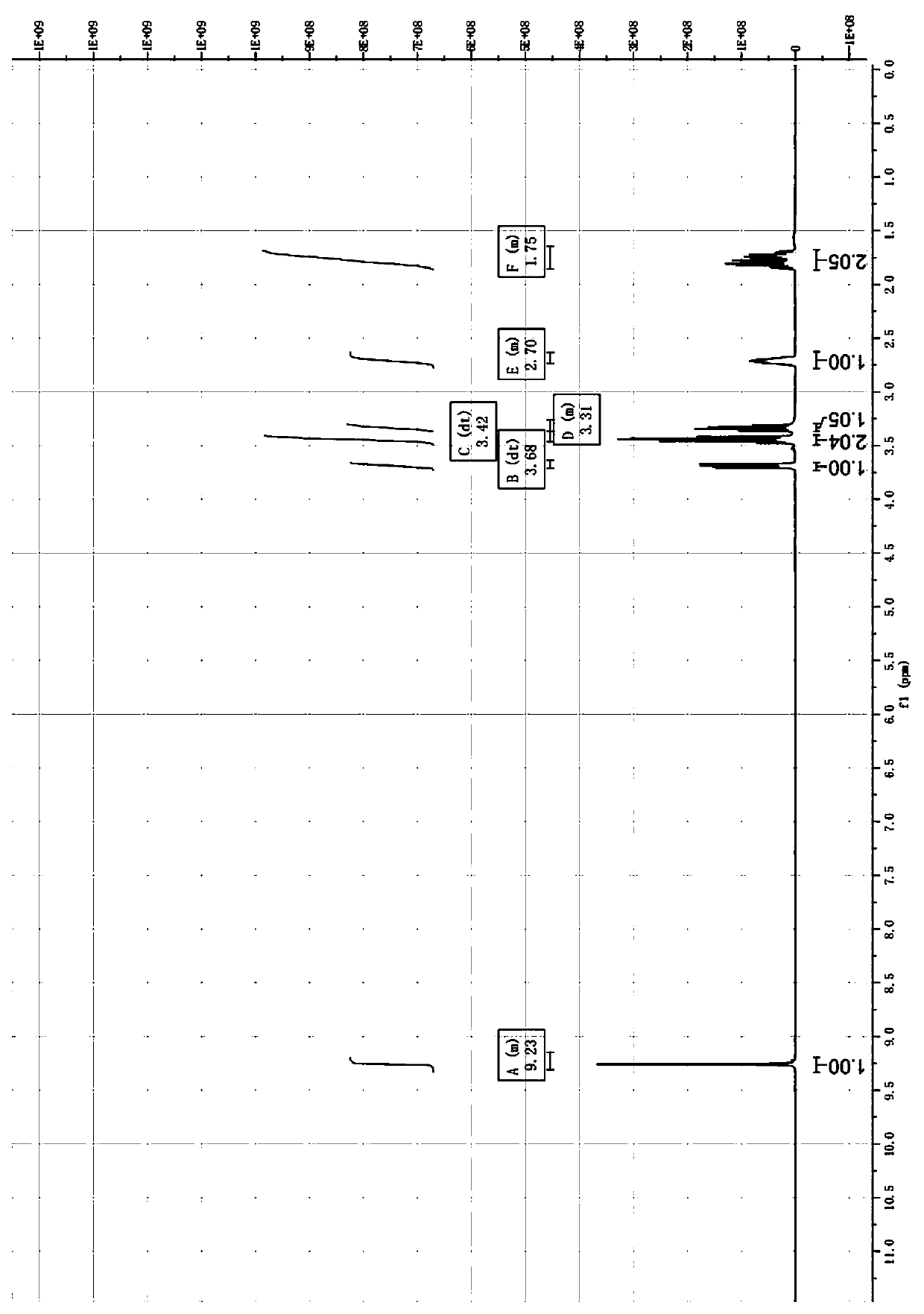
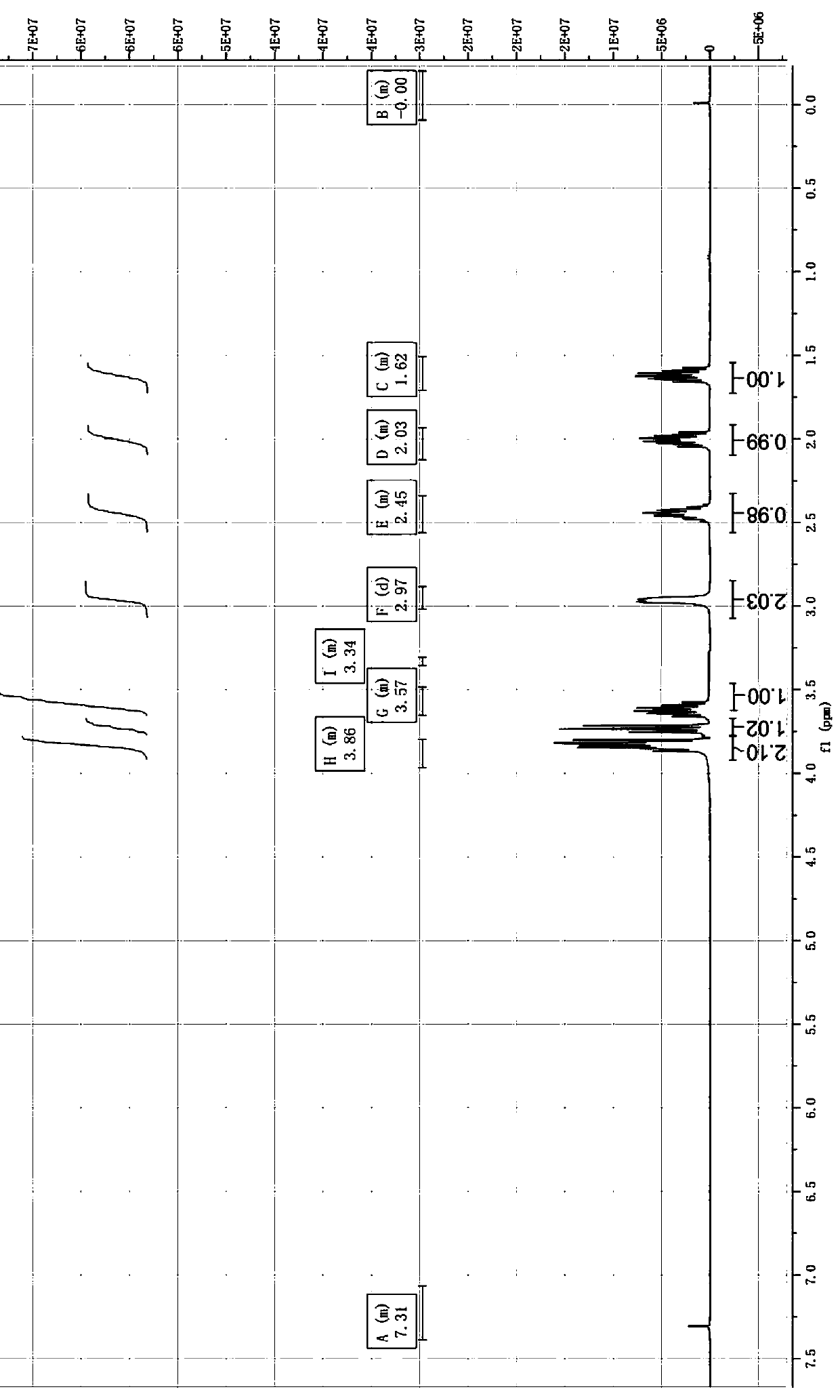
Preparation method of 3-aminomethyl tetrahydrofuran
A technology of aminomethyltetrahydrofuran and formyltetrahydrofuran, which is applied in the field of preparation of chemical raw material medicine intermediates, can solve the problems of difficult operation, high cost, flammability and safety of palladium carbon, etc., and achieve large production capacity, high selectivity, easy The effect of industrialization amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add 100 g of modified montmorillonite to the fixed-bed reactor, keep the temperature in the reactor at 120° C., feed maleic glycol at a flow rate of 0.30 L / h, and stop feeding when the feed rate reaches 3000 g. Then the temperature was raised to 150°C at 1°C per minute, and 2,5-dihydrofuran was collected by distillation. Under the catalysis of modified montmorillonite, maleic diol was continuously ring-closed and dehydrated to generate 2,5-dihydrofuran , by continuously collecting the product, the reaction proceeded in the positive direction. The reaction took 6 hours, and a total of 2,5-dihydrofuran was collected. 2050g, the yield was 86.1%, and the chemical purity was 99.5%.
Embodiment 2
[0059] Add 100 g of hydroxyapatite to the fixed-bed reactor, keep the temperature in the reactor at 120° C., feed maleic glycol at a flow rate of 0.30 L / h, and stop feeding when the amount reaches 5000 g. Then, after raising the temperature at 1°C per minute to 150°C, start to collect 2,5-dihydrofuran by distillation. Under the catalysis of hydroxyapatite, maleic diol is continuously ring-closed and dehydrated to generate 2,5-dihydrofuran. By continuously collecting the product, the reaction proceeded in the positive direction. The reaction took 12 hours, and a total of 3833 g of 2,5-dihydrofuran was collected, with a yield of 96.7% and a chemical purity of 99.3%.
Embodiment 3
[0061] Add 100 g of alumina to the fixed bed reactor, keep the temperature in the reactor at 120°C, feed maleic diol at a flow rate of 0.30 L / h, and stop feeding when the amount reaches 4000 g. Then the temperature was raised to 150°C at 1°C per minute, and 2,5-dihydrofuran was collected by distillation. Under the catalysis of alumina, maleic diol was continuously dehydrated to form 2,5-dihydrofuran through ring closure. The product was collected, and the reaction proceeded in the positive direction. The reaction took 10 hours, and a total of 2,5-dihydrofuran was collected, 2933g, with a yield of 92.4% and a chemical purity of 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com