Preparation method of aluminum acetylacetonate
A technology of aluminum acetylacetonate and acetylacetone, which is applied in the field of preparation of aluminum acetylacetonate, can solve the problems of large amount of acetylacetonate, large consumption of organic solvents, and low product purity, so as to promote the reaction, reduce the generation of waste water, and save solvents the effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
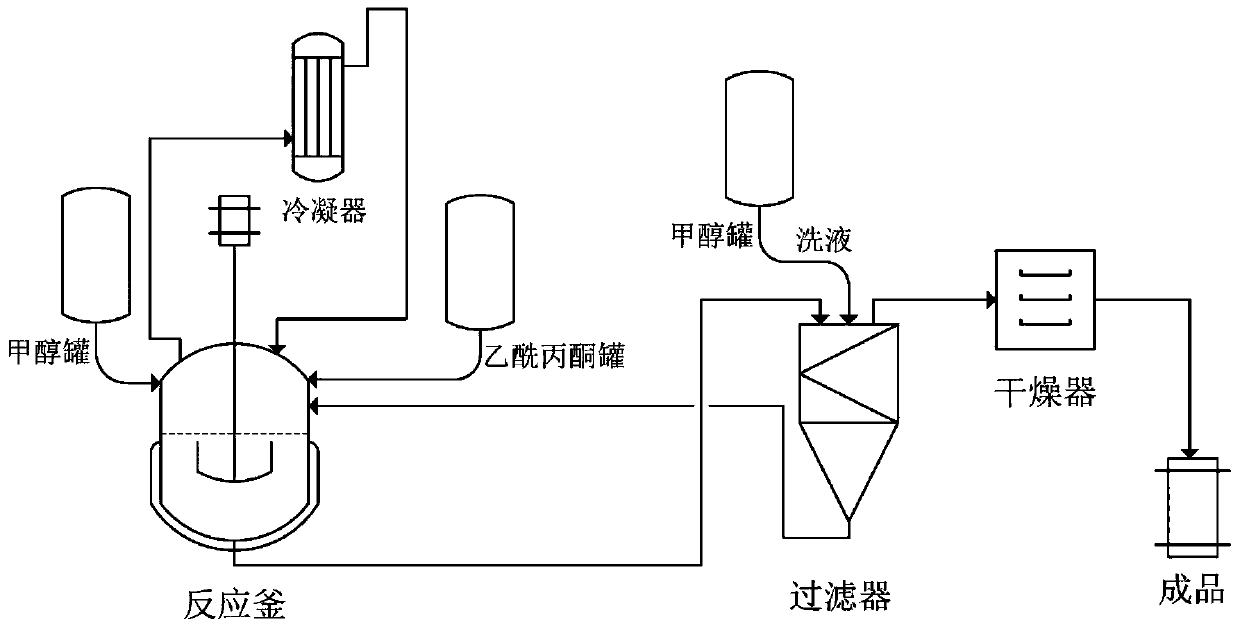
[0038] This example prepares the technological process schematic diagram of aluminum acetylacetonate to see attached figure 1 . Refer below figure 1 , illustrate that this example prepares the method for aluminum acetylacetonate as follows:
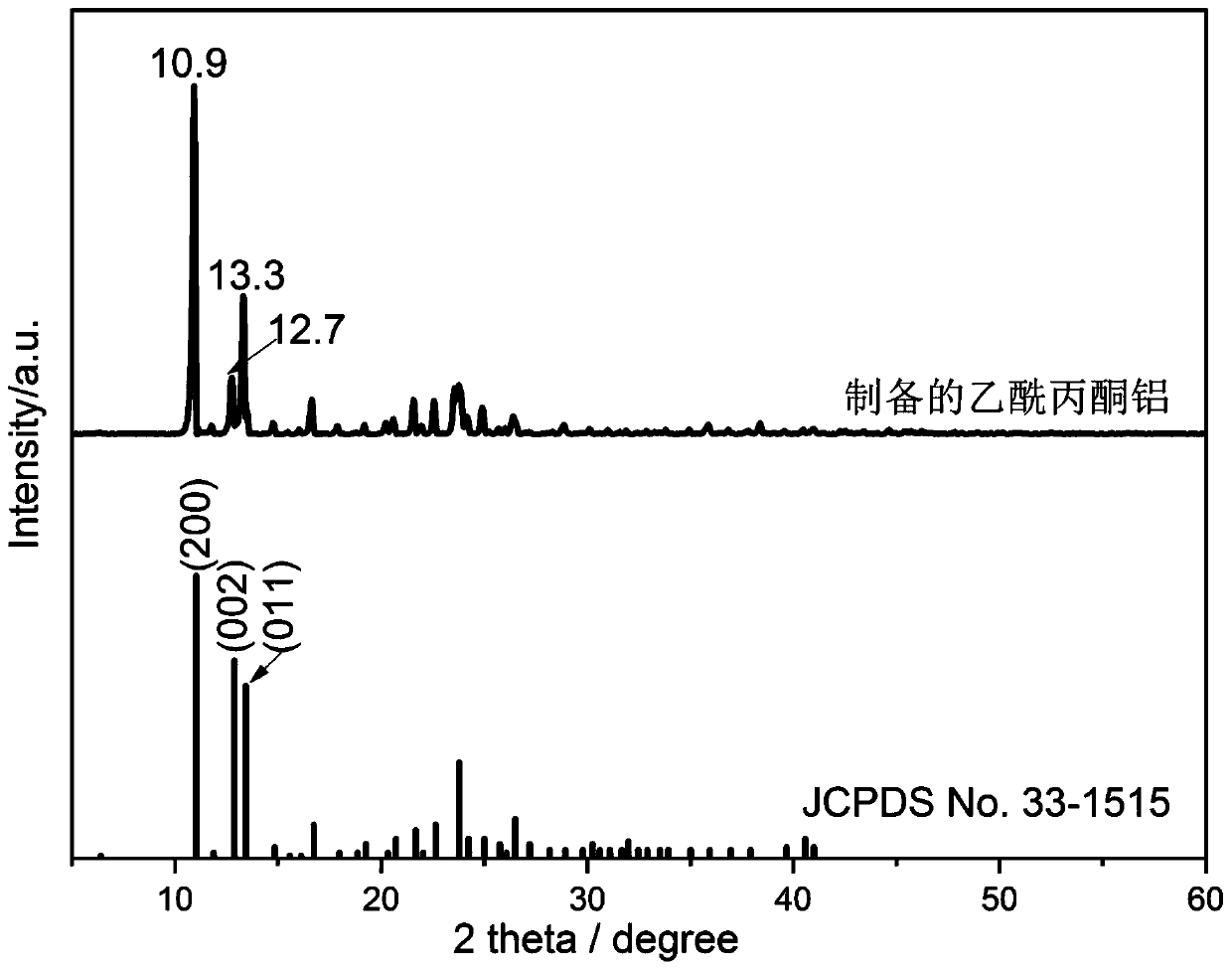
[0039] Weigh 2.0g of aluminum powder and place it in the reactor, and add 50mL of methanol into the reactor from the methanol tank. Turn on the mixer to mix the aluminum powder evenly. Heat the reaction system to 65° C., maintain the temperature until the end of the reaction, and use formic acid to adjust the pH of the reaction solution to 3. Add 30.3mL of acetylacetone from the acetylacetone tank (the molar ratio of acetylacetone to aluminum powder is 4:1), drop it into the reaction kettle at a rate of 1-2 drops per second, until the aluminum powder is completely reacted (no Solid exists, and solvent can be replenished, and the reaction time at this stage is 12h). The reaction was continued for 4h, and the total reaction time was 16...
Embodiment 2
[0043] The preparation method of this example aluminum acetylacetonate is as follows:
[0044] Weigh 2.0g of aluminum powder and place it in the reaction kettle, and add 60mL of ethanol from the ethanol tank as a solvent. Turn on the mixer to mix the aluminum powder evenly. Heat the reaction system to 75° C., maintain the temperature until the end of the reaction, and use acetic acid to adjust the pH of the reaction solution to 4.0. Add 37.9 mL of acetylacetone from the acetylacetone tank (the molar ratio of acetylacetone to aluminum powder is 5:1), and drop it into the reaction bottle at a rate of 1-2 drops per second until the aluminum powder is completely reacted (no Solid exists, and the reaction time at this stage is 10h). The reaction was continued for 4h, and the total reaction time was 14h. Stop heating and cool to 30°C, add dropwise 2mol / L NaOH solution to adjust the pH to 7, so that aluminum acetylacetonate crystallizes out. The reaction solution was filtered, an...
Embodiment 3
[0047] The preparation method of this example aluminum acetylacetonate is as follows:
[0048] Weigh 2.0g of aluminum powder and place it in the reaction kettle, and add 60mL of ethanol from the ethanol tank as a solvent. Turn on the mixer to mix the aluminum powder evenly. Heat the reaction system to 75° C., maintain the temperature until the end of the reaction, and use formic acid to adjust the pH of the reaction solution to 3. Add 37.9mL of acetylacetone from the acetylacetone tank (the molar ratio of acetylacetone to aluminum powder is 5:1), drop it into the reaction bottle at a rate of 1-2 drops per second, and continuously add formic acid during the reaction to maintain the pH at 2 to 3, until the aluminum powder is completely reacted (no solid is observed in the reaction solution, and the reaction time at this stage is 14 hours). The reaction was continued for 4h, and the total reaction time was 18h. Stop heating and cool to 28°C, add dropwise 3mol / L NaOH solution t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com