Aromatic heterocyclic compound 2,4,6,8-tetraamino-1,5-naphthalenediol hydrochloride and synthesis method thereof
A technology of naphthalene diol hydrochloride and synthesis method, which is applied in the field of organic compound preparation, can solve the problems of harsh synthesis conditions, high cost, poor performance, etc., and achieve fast reaction speed, high conversion rate, and improved chemical resistance stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
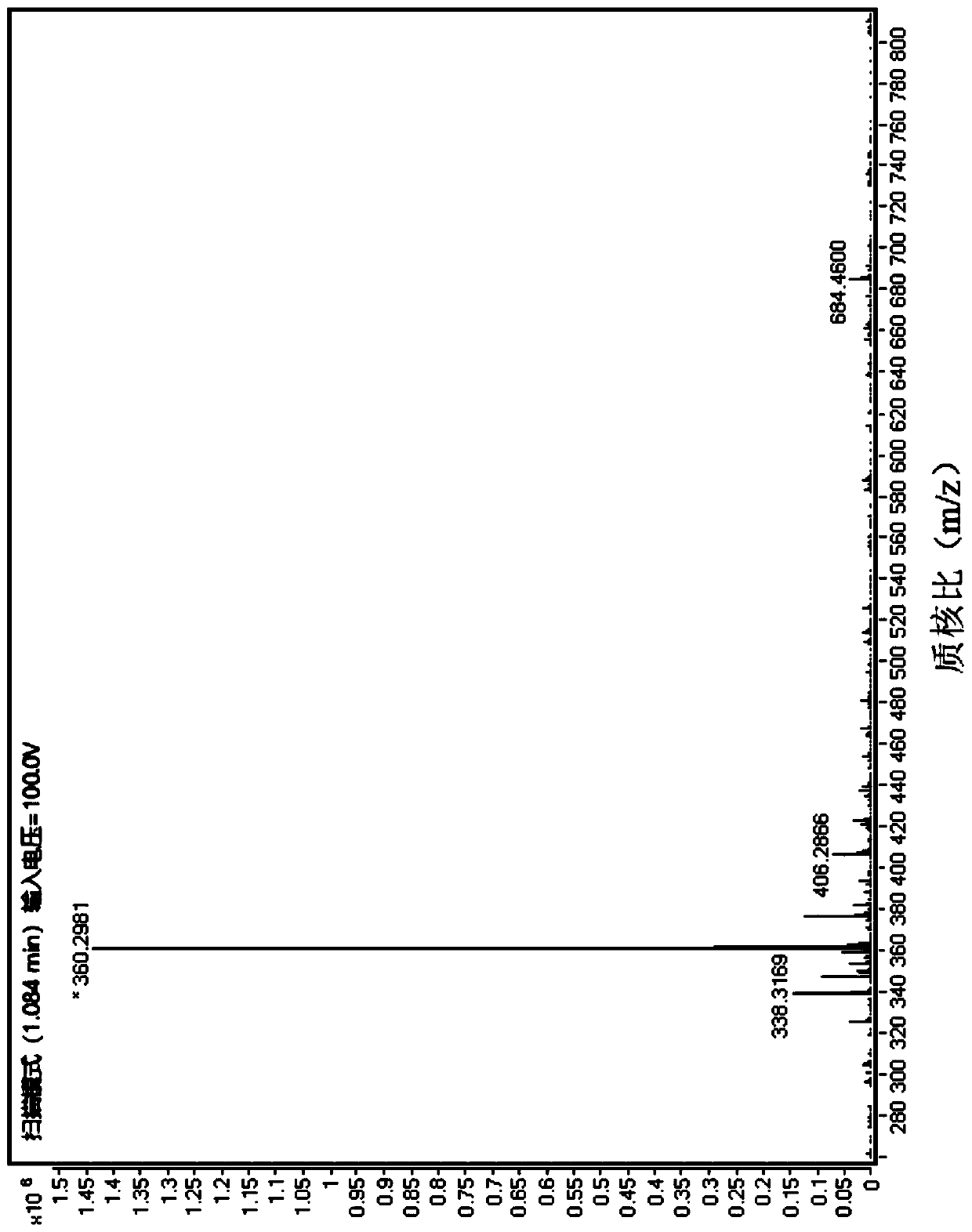
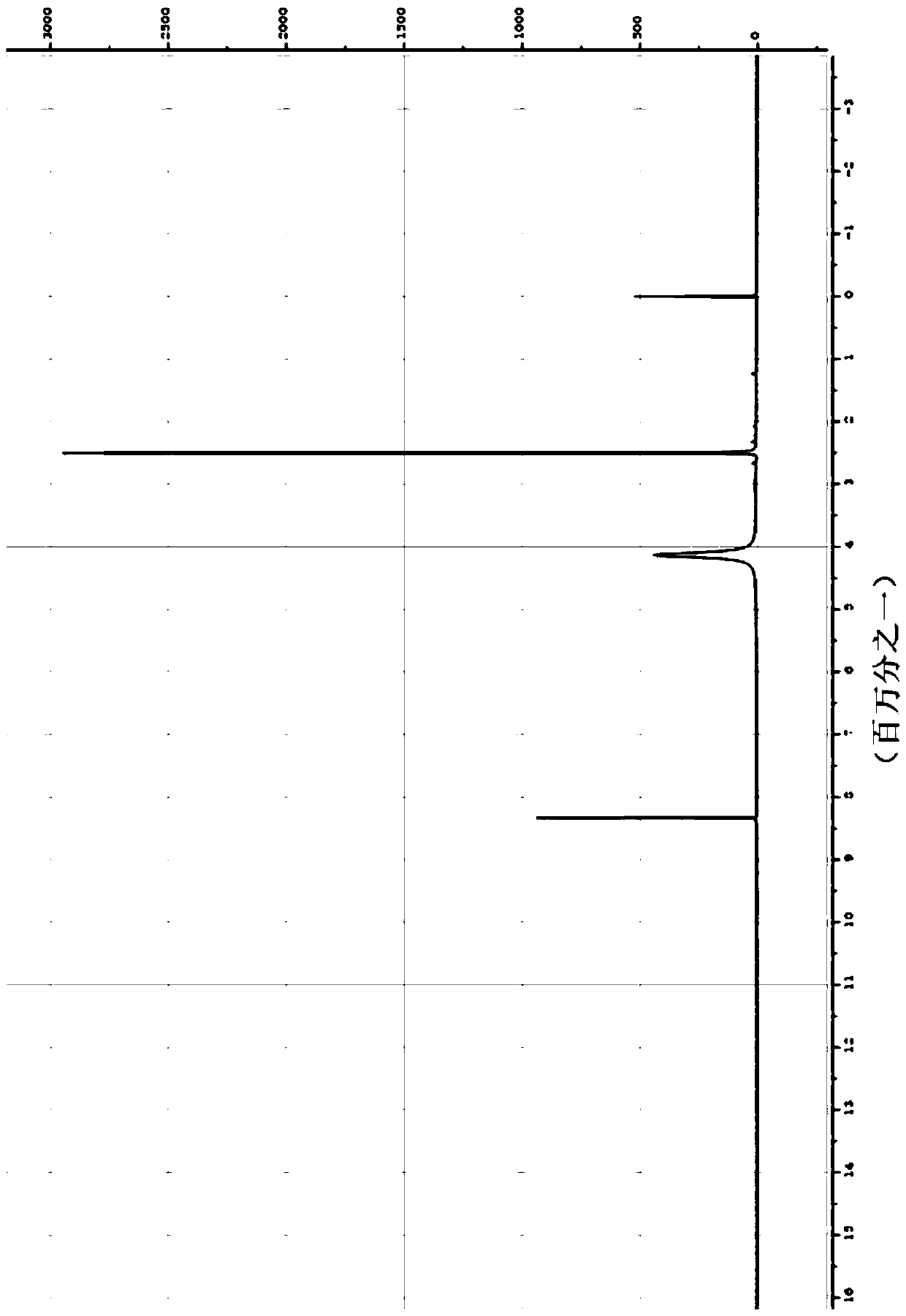
[0018] Specific embodiment one: In this embodiment, a kind of aromatic heterocyclic compound 2,4,6,8-tetraamino-1,5-naphthalenediol hydrochloride, its chemical formula is: C 10 h 16 Cl 4 N 4 o 2 ; Its molecular formula is:
specific Embodiment approach 2
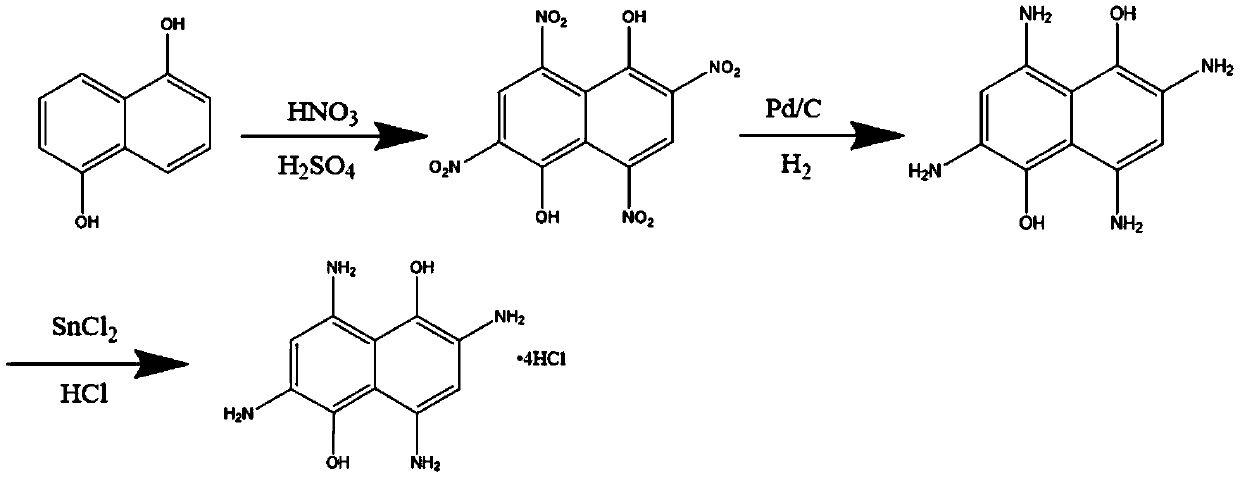
[0019] Specific embodiment two: In this embodiment, a method for synthesizing aromatic heterocyclic compound 2,4,6,8-tetraamino-1,5-naphthalenediol hydrochloride is realized according to the following steps:
[0020] 1. In a three-necked flask, dissolve 1,5-naphthalenediol in concentrated sulfuric acid with a mass concentration of 80-98%, then add mixed acid dropwise, and obtain 2,4,6 after stirring reaction, suction filtration, water washing, and drying. , 8-tetranitro-1,5-naphthalenediol crude product, redissolved in an organic solvent for recrystallization to obtain bright yellow needle crystals, collected by suction filtration to obtain 2,4,6,8-tetranitro - Pure 1,5-naphthalenediol;
[0021] 2. In a high-pressure reactor, the pure product of 2,4,6,8-tetranitro-1,5-naphthalenediol is dissolved in absolute ethanol as a substrate, and then a Pd / C catalyst is added for hydrogenation reaction, After the reaction is completed, filter, remove the Pd / C catalyst, and then pass int...
specific Embodiment approach 3
[0024] Embodiment 3: This embodiment is different from Embodiment 2 in that the weight ratio of 1,5-naphthalenediol to concentrated sulfuric acid with a mass concentration of 80-98% in step 1 is 1:(2-10). Other steps and parameters are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com