Adeno-associated virus (AAV) containing variant capsid protein and application of AAV
A capsid protein and virus technology, applied in the field of adeno-associated virus, can solve the problems of low infection efficiency and lack of support cells, and achieve the effect of good technical support and efficient infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Constructing AAV variants and infecting HEK 293T cells
[0062] Construction of AAV variant (named AAV-ie) Rep-Cap plasmid.
[0063] The packaging of AAV requires three plasmids: a genome plasmid containing the gene of interest, a Rep-Cap plasmid, and a Helper plasmid.
[0064] The sequence of the Cap protein in the Rep-Cap plasmid determines the different serotypes of AAV, which in turn affects the preference of AAV-infected cells. Therefore, modification of the Cap protein can lead to new AAV types.
[0065] (1) Construction, digestion and purification of the vector
[0066] The Rep-Cap plasmid of parental AAV-DJ was synthesized by Nanjing Genscript Company (www.genscript.com.cn).
[0067] First, a unique NheI endonuclease site was introduced between amino acids 589 and 590 of the wild-type AAV-DJVP1 capsid protein by polymerase chain reaction (PCR) mutagenesis, with primers from Genscript Nanjing (www.genscript.com. cn) synthesis. It was then digested ...
Embodiment 2
[0079] Example 2 AAV variant infects mouse cochlear tissue in vitro
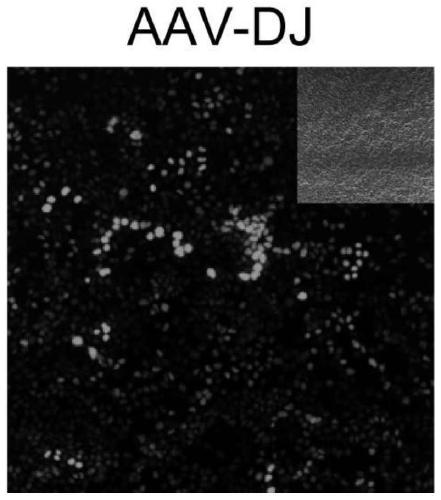
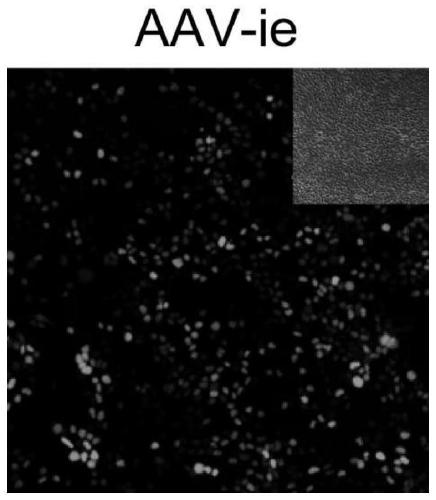
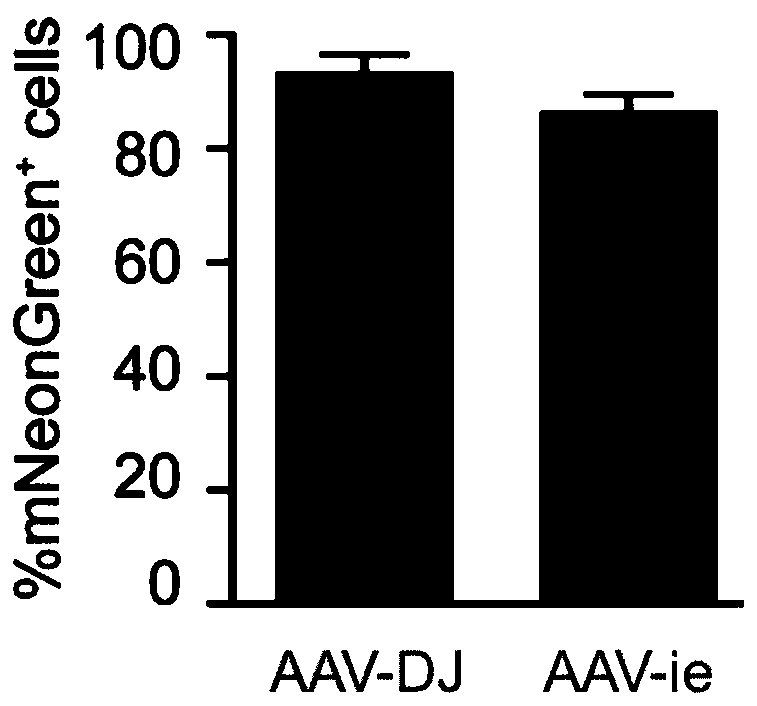
[0080] Quickly take out the cochlea of P3 wild-type C57 mouse (Shanghai Lingchang Biotechnology Co., Ltd.) on ice, paste it on the glass slide coated with cell-tak, and place it in 98% DMEM+1%N 2 +1%Amp (5ug / mL) After stabilizing in the medium for 12h, add 1%FBS and 2×10 10 GC AAV cultured for 48-60h. The cultured samples were then identified by immunostaining. Samples were fixed by immersing in 4% medium PFA, then immersed in 10% donkey serum and 0.3% Triton X-100PBS, incubated at room temperature for 1 hour, added Myo7a (myosin 7a), Sox2 protein antibodies and Corresponding secondary antibodies. The samples were mounted with anti-fluorescent quencher mounting medium and observed by confocal.
[0081] When capturing images using the confocal method, choose the laser power setting for capturing variant-infected samples as standard. All visible green fluorescent protein mNeonGreen signals were captured...
Embodiment 3
[0086] Example 3 AAV variants can efficiently infect various tissue cells in mouse cochlea after in vivo injection
[0087] Using the cochlear round window injection technique, 1.5 uL of AAV variant virus (concentrations of various viruses are marked in Figure 4) was injected into the perilymph of the cochlea. The specific steps are as follows: The method of anesthesia for neonatal mice adopts the method of low temperature induction anesthesia. P2-3 mice were placed in an ice bath for 2-3 minutes, and then taken out on an ice pad for subsequent surgical procedures. Surgery was performed only in the left ear of each mouse, and the right ear served as a negative control. During the operation, an incision was made behind the left ear, and the round window was exposed according to the relative position of the temporal bone and the facial nerve. Be careful not to damage the facial nerve during the operation. Next, AAV was injected into the cochlea from the round window through a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com