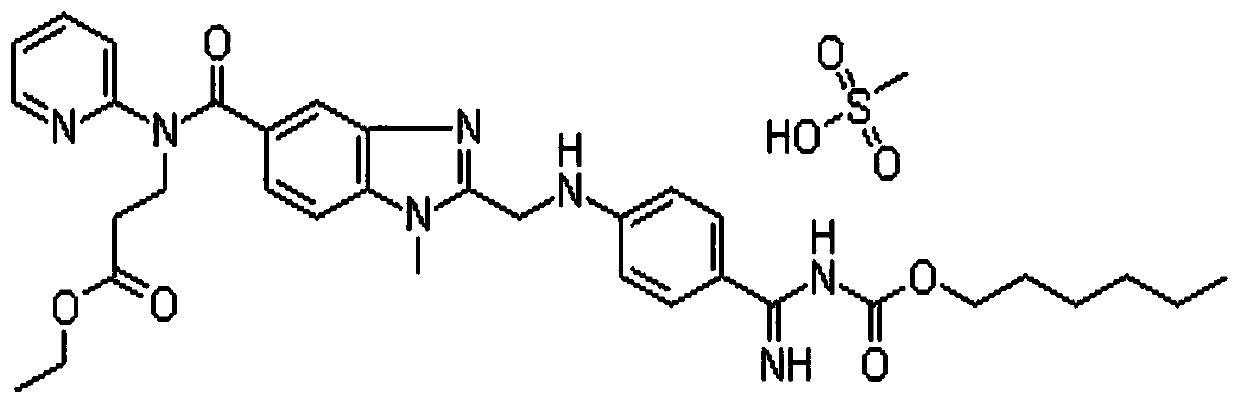
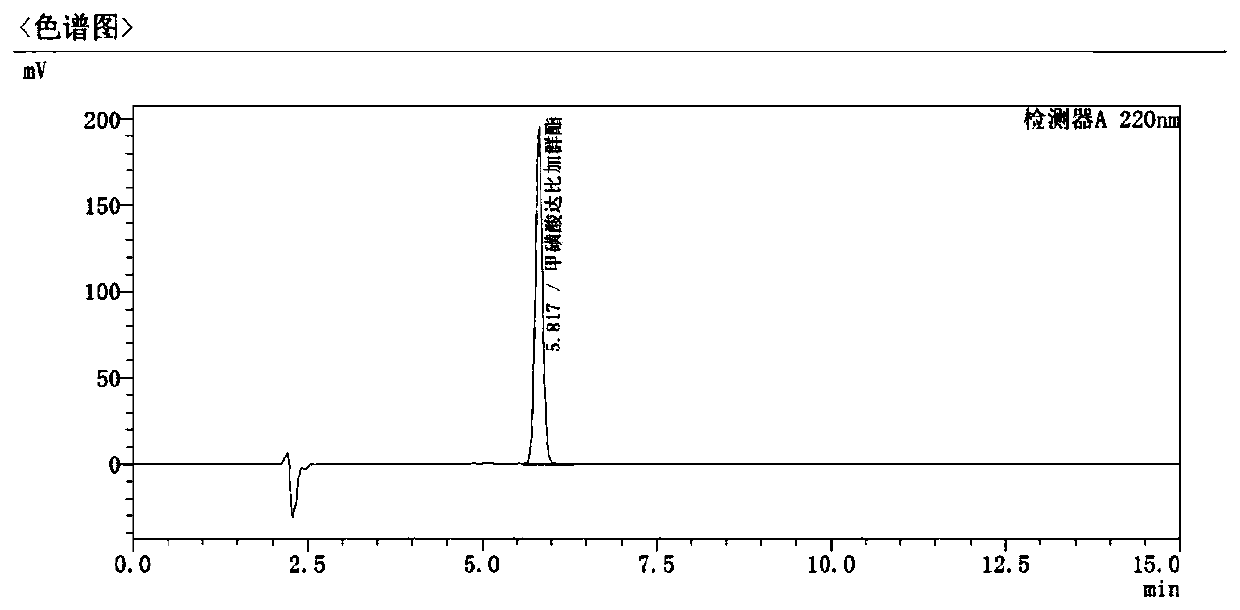
Method for detecting dabigatran etexilate mesylate
A technology of dabigatran etexilate mesylate and a detection method, applied in the field of medicine, can solve the problem of no dabigatran etexilate mesylate and the like, and achieve a high tailing factor, good system applicability and high precision. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: use the method for high performance liquid chromatography to detect dabigatran etexilate mesylate content
[0033] Take 1L of purified water, add 0.02mol ammonium acetate (1.54g), dissolve and add triethylamine to adjust the pH to 8.0, take 700ml of the above solution, add 1300ml of acetonitrile, mix well, and filter to obtain the mobile phase;
[0034] Take 500ml of acetonitrile, add 500ml of purified water, mix well, and prepare a diluted solution;
[0035] Take about 20mg of dabigatran etexilate mesylate, accurately weigh it, put it in a 100ml measuring bottle, add acetonitrile water (1:1) to dissolve and dilute to the mark, shake well, accurately measure 5ml and put it in a 50ml measuring bottle, add Acetonitrile water (1:1) is diluted to scale, shakes up, makes need testing solution;
[0036] Get about 20mg of dabigatran etexilate mesylate reference substance, accurately weighed, put in a 100ml measuring bottle, add acetonitrile water (1:1) to dissol...
Embodiment 2
[0039] Embodiment 2: Ammonium acetate solution and acetonitrile ratio investigation
[0040] Three mobile phases with volume ratios of 10:90, 35:65, and 70:30 were prepared using the solution containing 0.02 mol / L ammonium acetate and acetonitrile prepared according to Example 1. The rest of the conditions are the same as in Example 1, and the resolution, main peak retention time and impurity peak quantity of different mobile phases are shown in Table 1.
[0041] Table 1 The resolution, main peak retention time and impurity peak quantity of different mobile phase ratios
[0042] Ammonium Acetate: Acetonitrile Main peak retention time (min) Separation Number of impurity peaks 10:90 3.533 1.632 3 35:65 5.817 2.331 4 70:30 9.212 1.965 4
[0043] It can be seen that with the decrease of the organic phase in the mobile phase, the retention time of the main peak is extended, and the separation degree of the main peak and adjacent impurities ...
Embodiment 3
[0044] Embodiment 3: the investigation to the concentration of ammonium acetate solution
[0045] Take 1L of purified water, add 0.01mol (0.77g), 0.02mol (1.54g) and 0.03mol (2.31g) of ammonium acetate, after dissolving, add triethylamine to adjust the pH to 8.0, take 350ml of the above solution, mix it with 650ml of acetonitrile, Three mobile phases were prepared separately. All the other conditions are the same as in Example 1. The resolution, retention time of main peak and number of impurity peaks of different mobile phases are shown in Table 2.
[0046] The degree of separation and the retention time of the main peak and the number of impurity peaks of different ammonium acetate solution concentrations in table 2
[0047]
[0048] As the concentration of ammonium acetate increases, the retention time of the main peak shortens, and the resolution of the main peak and adjacent impurity peaks decreases. When the concentration is 0.02mol / L, the retention time of the main...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com