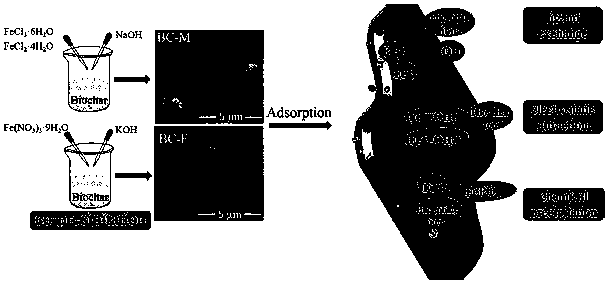
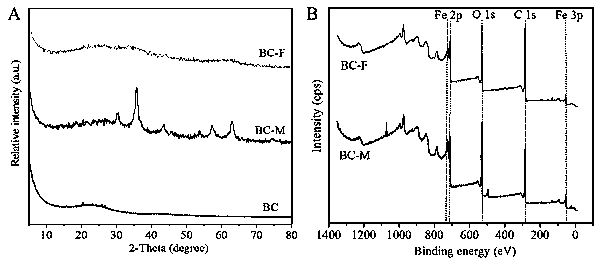
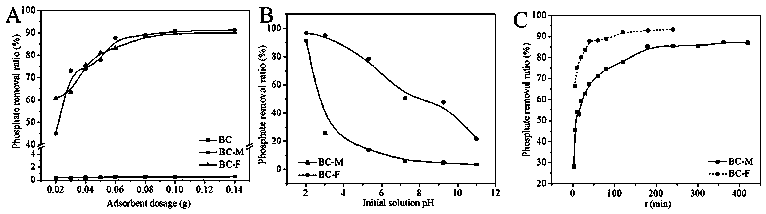
Preparation method and application of biochar/iron oxide composite material
A technology of composite materials and iron oxides, applied in chemical instruments and methods, water pollutants, other chemical processes, etc., can solve problems such as restriction removal, achieve good effect and good effect of repeated use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Pass the fruit shell biochar pyrolyzed at 900°C through a 200-mesh sieve.
[0041] (2) Weigh 5 g of fruit shell biochar, 2.7 g of ferric chloride hexahydrate and 1.0 g of ferrous chloride tetrahydrate into a 250 mL beaker, dissolve in 100 mL of distilled water, and record it as solution A; prepare 1 mol of / L NaOH solution, denoted as solution B.
[0042] (3) The solution A was sonicated for 0.5 h until it was evenly mixed, then added to a four-necked flask, and stirred for 1.5 h at an ambient temperature of 60 °C, a rotational speed of 140 r / min, and a nitrogen atmosphere. Add solution B dropwise to the suspension in the four-neck flask, adjust the pH to about 10, and stir at 90°C for 1.5 h.
[0043] (4) After the reaction is completed, the mixed solution is washed until neutral, and the obtained precipitate is freeze-dried for later use.
[0044]At room temperature, 0.06 g of biochar / magnetite composite material was added to 20 mL of 8 mg / L phosphate solution, s...
Embodiment 2
[0046] (1) Pass the fruit shell biochar pyrolyzed at 900°C through a 200-mesh sieve.
[0047] (2) Weigh 5 g of fruit shell biochar, 7.6 g of ferric chloride hexahydrate and 2.8 g of ferrous chloride tetrahydrate into a 250 mL beaker, dissolve with 100 mL of distilled water, and record it as solution A; prepare 1 mol of / L NaOH solution, denoted as solution B.
[0048] (3) The solution A was sonicated for 0.5 h until it was evenly mixed, then added to a four-necked flask, and stirred for 1.5 h at an ambient temperature of 60 °C, a rotational speed of 140 r / min, and a nitrogen atmosphere. Add solution B dropwise to the suspension in the four-neck flask, adjust the pH to about 10, and stir at 90°C for 1.5 h.
[0049] (4) After the reaction is completed, the mixed solution is washed until neutral, and the obtained precipitate is freeze-dried for later use.
[0050] At room temperature, 0.06 g of biochar / magnetite composite material was added to 20 mL of 8 mg / L phosphate solution...
Embodiment 3
[0052] (1) Pass the fruit shell biochar pyrolyzed at 900°C through a 200-mesh sieve.
[0053] (2) Weigh 5 g of fruit shell biochar and 2.5 g of ferric nitrate nonahydrate into a 250 mL beaker, add 50 mL of distilled water, and stir with high-speed magnetic force for 0.5 h. Add 1 mol / L KOH dropwise to the above mixed solution, adjust the pH to about 7, and continue stirring for 0.5 h.
[0054] (3) After the reaction is completed, the mixed solution is washed until neutral, and the obtained precipitate is vacuum-dried at 60°C for later use.
[0055] At room temperature, 0.05 g of biochar / ferrihydrite composite material was added to 20 mL of 8 mg / L phosphate solution, shaken for 4 h, and the adsorption rate reached 15.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com