Preparation method of L-menthol
A technology of menthol and menthyl, applied in the preparation field of L-menthol, can solve problems such as low ee value of L-menthyl benzoate, industrialized yield, large solvent consumption, etc., to avoid intermittent crystallization mode, The effect of improving production efficiency and reducing the use of solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] A kind of preparation technology of embodiment 1 L-menthol
[0049] Include the following steps:
[0050] (1) Preparation of D,L-menthyl benzoate
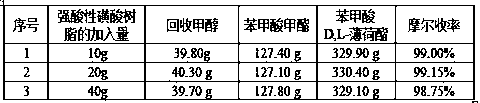
[0051] Add 200.0g of D,L-menthol, 300.0g of methyl benzoate, 10g-40g of strong acidic sulfonic acid resin to a 1000ml three-necked flask, and pull a vacuum on the reaction system. Control the vacuum degree at 2000pa-10000pa and control the reaction temperature at 80 ℃, reacted for 5 hours, and the light components after the reaction under vacuum are more conducive to timely separation, which greatly accelerates the speed of the reaction. After filtration, the filtrate enters the rectification system, and the resin is recovered mechanically; The methyl benzoate that has reacted, D, L-menthyl benzoate, the molar yield of this step is shown in the following table:
[0052]
[0053] (2) split
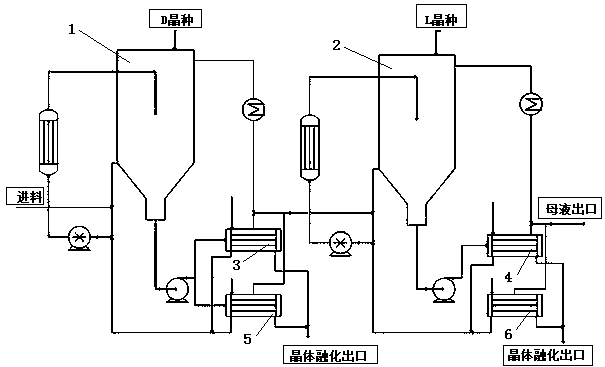
[0054] as attached figure 1 As shown, add 5 L each of D benzoate and L-menthyl to the primary melting crystallizer 1 and secondar...
Embodiment 2
[0079] A kind of preparation technology of embodiment 2 L-menthol
[0080] Include the following steps:
[0081] (1) Preparation of D,L-menthyl phenylacetate
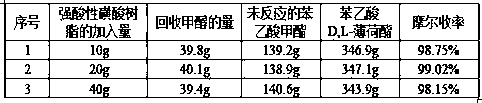
[0082] Add 200.0g of D,L-menthol, 330.0g of methyl phenylacetate, 10-40g of strong acidic sulfonic acid resin into a 1000ml three-necked flask, and pull a vacuum on the reaction system. ℃, reacted for 5 hours, and the light components after the reaction under vacuum are more conducive to timely separation, which greatly speeds up the reaction speed. After filtration, the filtrate enters the rectification system, and the resin is recovered and applied mechanically; Finished methyl phenylacetate, D, L-menthyl phenylacetate, the actual molar yield of this step is shown in the following table:
[0083]
[0084] (2) split
[0085] as attached figure 1 As shown, add 10L of D,L-menthyl phenylacetate to the primary melting crystallizer 1 and the secondary melting crystallizer 2 (each crystallizer has a volume of 5L, just o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com