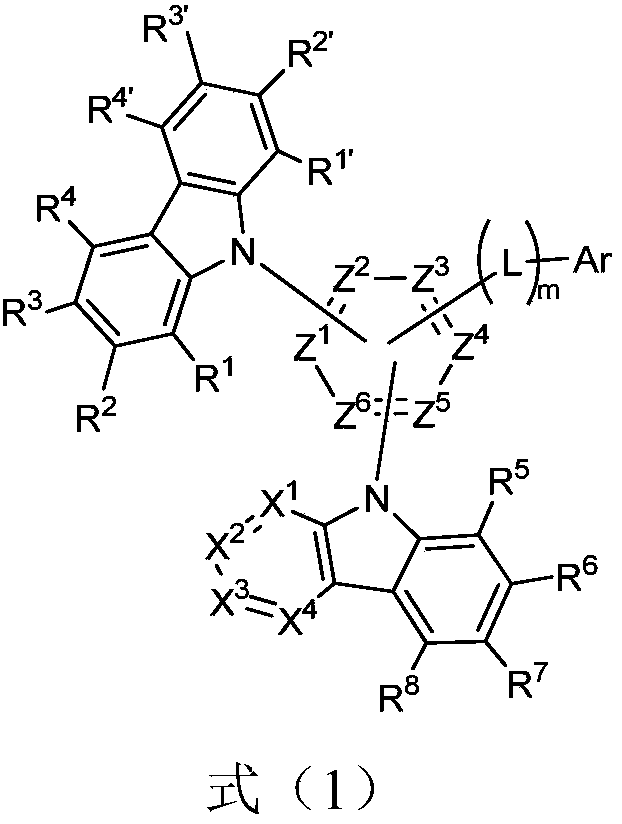
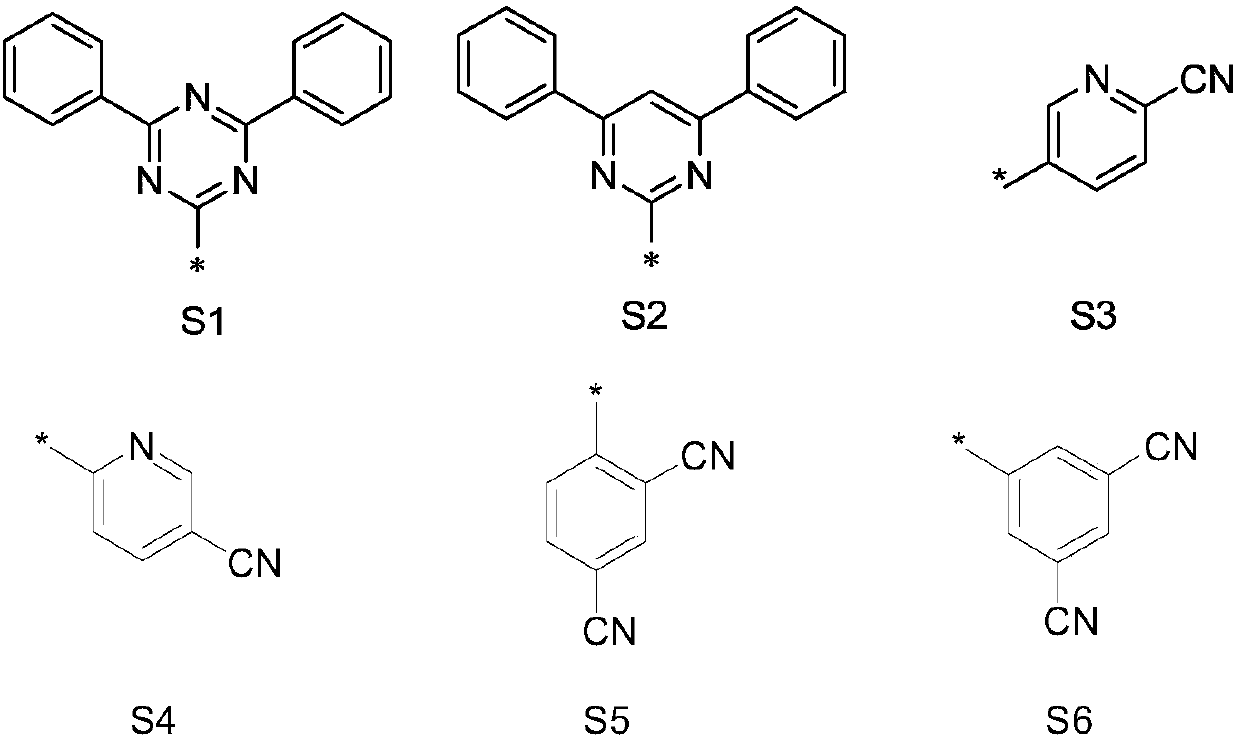
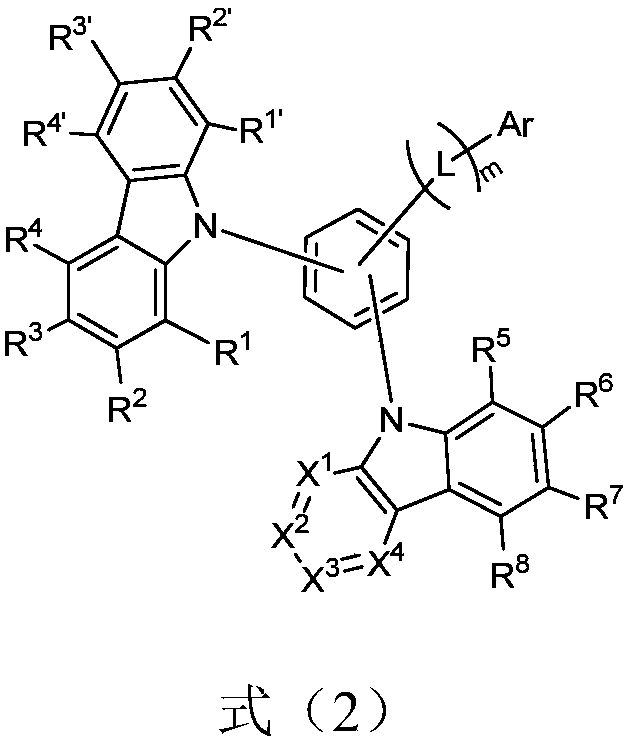
Nitrogen-containing heterocyclic compound, application thereof and organic light-emitting device
A nitrogen heterocyclic compound and heterocyclic compound technology, which is applied in the field of organic electroluminescent materials, can solve the problem of unbalanced electron and hole transport, severe triplet-polaron annihilation, and low crossover rate between inverse systems, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0079] Preparation of intermediate M1:
[0080] Add 13.5g (70mmol, 1eq) of 2,5-difluorobromobenzene, 35.4g (140mmol, 2eq) of diboronic acid pinacol ester, and 68g (697mmol, 20%eq), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride 6.1g (8.4mmol, 12%eq), solvent dioxane 1000mL, add and replace nitrogen 3 times. The temperature of the oil bath was raised to 100° C., and the reaction was carried out overnight. (PE:EA=10:1, product Rf=0.5, 2,5-difluorobromobenzene Rf=1.0)
[0081] The reaction solution was lowered to room temperature, extracted with ethyl acetate, the upper layer was taken, the reaction solution was spin-dried, and PE:EA=30:1 was subjected to column chromatography to obtain a white solid, intermediate M1, 13g.
[0082] Preparation of intermediate M2:
[0083] Add toluene 500mL, intermediate M1 20g (83.3mmol, 1.2eq), 4-bromoisophthalonitrile 14.3g (70mmol, 1eq), sodium carbonate aqueous solution (sodium carbonate 22.3g, 210mmol, 3eq, water 105mL, 2M),...
Embodiment 2
[0181] Example 2 The organic electroluminescence performance of the OLED using the compound P28 of the present invention as a dye is better than that of the OLED organic electroluminescence performance using A129 as a dye in Comparative Example 1, and P28 obtains higher current efficiency and lower drive Voltage; This shows that the introduction of asymmetric carbazolecarboline groups into dyes to prepare organic electroluminescent devices can significantly reduce the driving voltage and improve the luminous efficiency.
[0182] At the same time, the organic electroluminescent performance of the OLED using the compound P66 of the present invention as the dye in Example 5 is better than that of the OLED using A130 as the dye in Comparative Example 2. P66 also obtained higher current efficiency and lower The driving voltage; this shows that the carboline group and the bridged pyridine group are introduced into the molecule, which can significantly reduce the driving voltage and i...
Embodiment 9
[0211] Example 9 The organic electroluminescent performance of the OLED using the compound P8 of the present invention as the main body is better than that of the OLED organic electroluminescent performance using CBP as the main body in Comparative Example 3, and the device in Example 9 has obtained higher current efficiency And lower driving voltage; This shows that the material based on asymmetric carbazolecarboline group is used as the main body to prepare organic electroluminescent devices, which can also obviously reduce the driving voltage and improve the advantages of luminous efficiency.
[0212] At the same time, the organic electroluminescence performance of the OLED using the compound P82 of the present invention as the dye in Example 11 is better than that of the OLED organic electroluminescence performance using P8 as the main body in Example 9. P82 has obtained higher current efficiency and lower Driving voltage; this shows that when the host material uses bridged...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com