A star-shaped molecular structure hindered phenolic compound and its application in the synthesis of polyester amide
A polyester amide and molecular structure technology, applied in the field of polyester amide resin, can solve the problems that antioxidants are difficult to achieve ideal effects, and the thermal stability and compatibility requirements of antioxidants are relatively high. Excellent performance and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
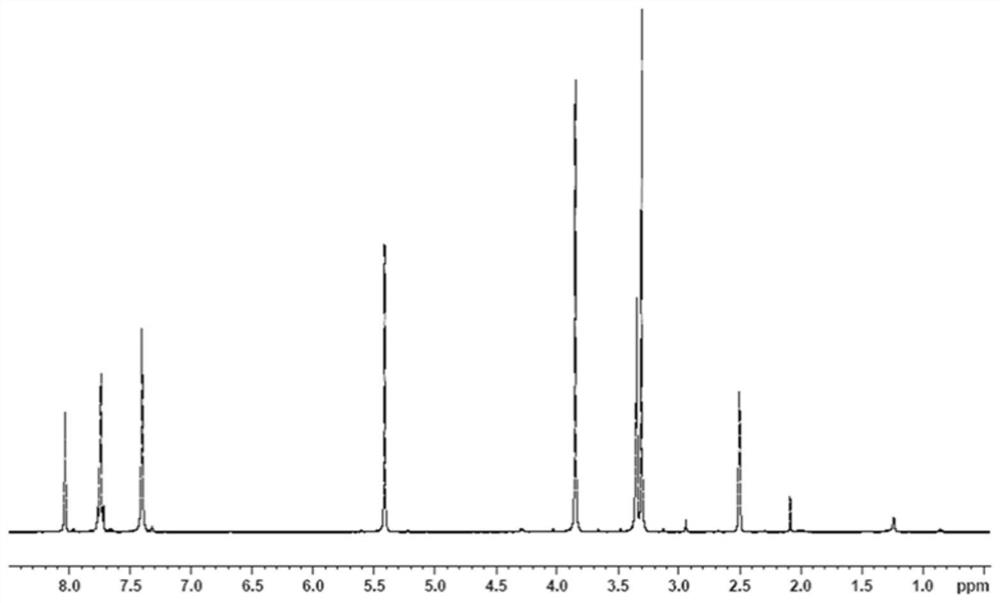
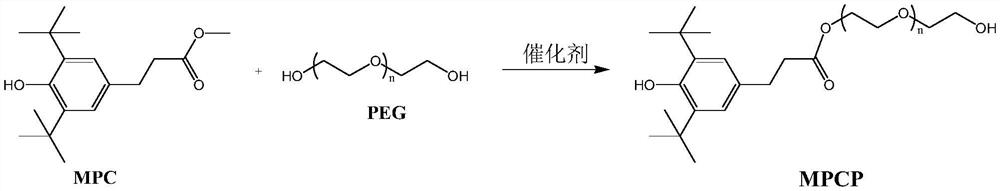
[0044] Synthetic compound MPCP (n=0)
[0045]Add 100G of methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate and 200G of ethylene glycol into the reaction flask. The temperature was raised to 40° C., stirring was started, and 0.45 g of tetraisopropyl titanate was added. The reaction temperature was raised to 75°C and distillate methanol began to collect. The reaction temperature was raised up to 95°C until there was no methanol distillate, and the reaction lasted for 5 hours. After the reaction was stopped, 200 mL of toluene was added to the reaction mixture, and the organic phase was washed three times with 300 mL of saturated brine, and once with 150 mL of pure water. The collected organic phase was concentrated by vacuum distillation to remove all volatile substances to obtain light yellow viscous liquid (ie compound MPCP) 110G.
[0046] Synthesis of Compound I (n=0)
[0047] 150 mL of dichloromethane, 13 G of TEA and 31 G of trimesoyl chloride were added to the reac...
Embodiment 2
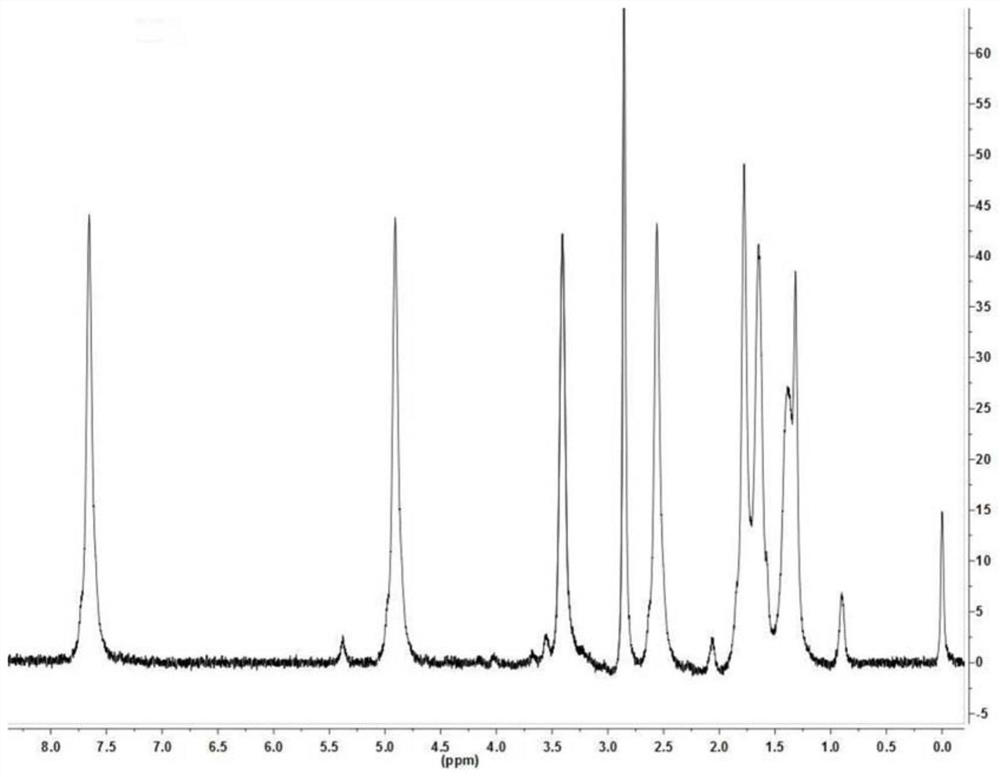
[0051] Synthetic compound MPCP(n=1)
[0052] Add 100G of methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate and 265G of diethylene glycol into the reaction flask. The temperature was raised to 40° C., stirring was started, and 0.45 g of tetrabutyl titanate was added. The reaction temperature was raised to 75°C and distillate methanol began to collect. The reaction temperature was raised up to 105°C until there was no methanol distillate, and the reaction lasted for 5 hours. After the reaction was stopped, 200 mL of toluene was added to the reaction mixture, and the organic phase was washed three times with 300 mL of saturated brine, and once with 150 mL of pure water. The collected organic phase was concentrated by vacuum distillation to remove all volatile substances to obtain light yellow viscous liquid (ie compound MPCP) 126G.
[0053] Synthesis of Compound I (n=1)
[0054] 150 mL of dichloromethane, 13 G of TEA and 31 G of trimesoyl chloride were added to the react...
Embodiment 3
[0058] Synthetic compound MPCP(n=2)
[0059] Add 100G of methyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate and 450G of triethylene glycol into the reaction flask. The temperature was raised to 40° C., stirring was started, and 0.75 g of tetraisopropyl titanate was added. The reaction temperature was raised to 75°C and distillate methanol began to collect. The reaction temperature was raised up to 110°C until no methanol distillate was present, and the reaction was carried out for a total of 6 hours. After the reaction stopped, 350 mL of toluene was added to the reaction mixture, and the excess triethylene glycol in the lower layer was separated under heating conditions (temperature 50° C.). The organic phase was washed once with 50 mL of pure water, and the collected organic phase was concentrated by vacuum distillation to remove all volatile substances to obtain a light yellow viscous substance (ie compound MPCP) 145G.
[0060] Synthesis of Compound I (n=2)
[0061] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| yellowness index | aaaaa | aaaaa |
| yellowness index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com