Zinc or zinc alloy electroplating method and system
A zinc alloy and electroplating bath technology, applied in the field of zinc or zinc alloy electroplating and systems, can solve problems such as inability to prolong the service life of the plating solution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
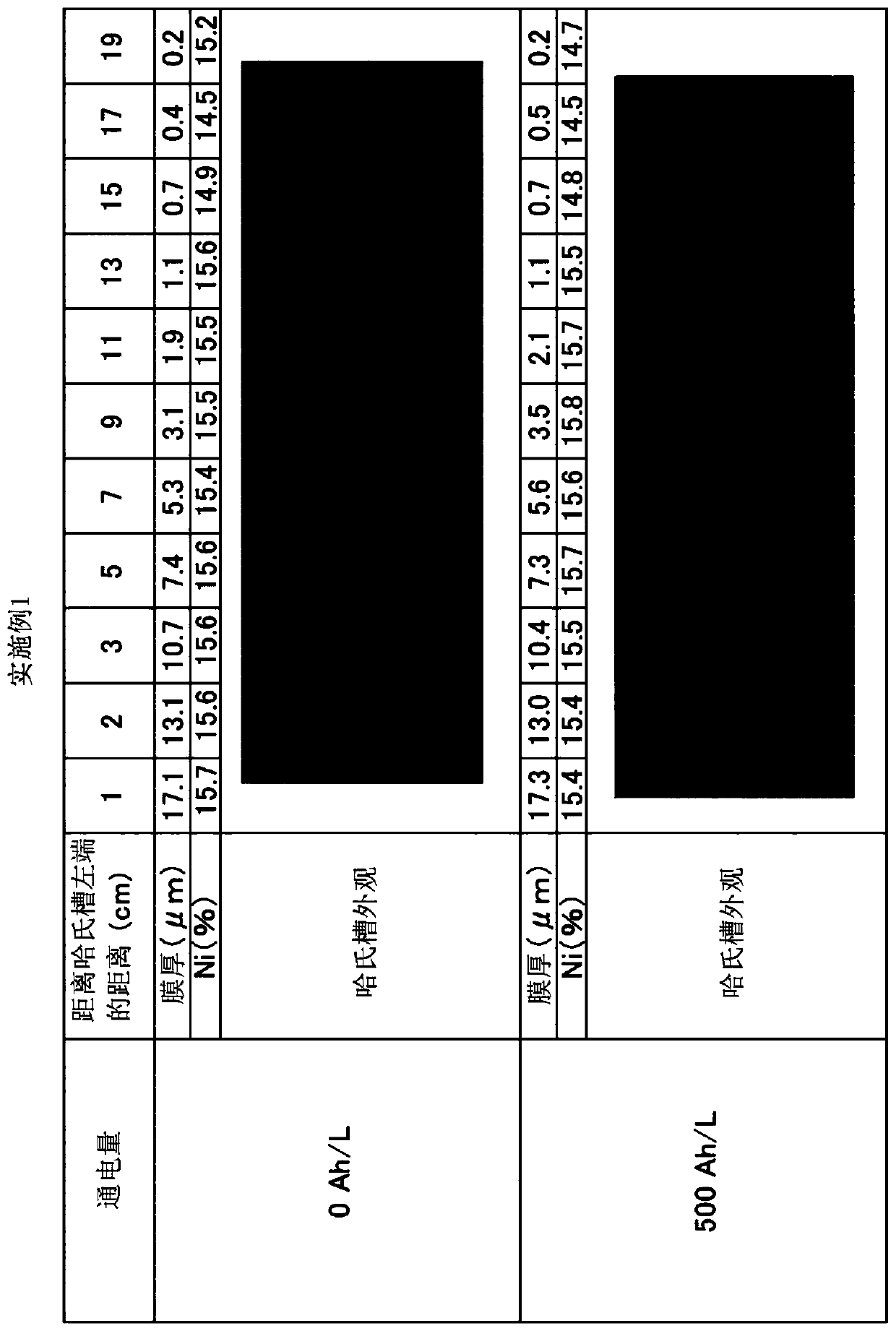
Embodiment 1
[0082] An anode plate (surface roughness Ra: 4 μm, 64×64×2 mm) coated with tantalum oxide on Ni with a thickness of 0.5 to 0.8 μm was used, and an alkaline zinc-nickel alloy plating bath (500 mL ), using 500Ah / L energization to implement zinc-nickel alloy plating. The pore diameter in the coating film was 0.1 to 1 μm, and the dipping out of the plating bath was 2 mL / Ah. The cathode current density is 4A / dm 2 , the anode current density is 9.8A / dm 2 , the plating bath temperature is 25°C. The bath was cooled and maintained at 25°C. An iron plate is used for the cathode. It should be noted that the iron plate of the cathode was replaced every 16Ah / L during energization. The concentration of zinc ions in the plating bath is maintained constant by dipping and dissolving metallic zinc. The concentration of nickel ions in the plating bath was kept constant by supplying nickel replenisher IZ-250YNi (manufactured by DIPSOL). The sodium hydroxide concentration of the plating bat...
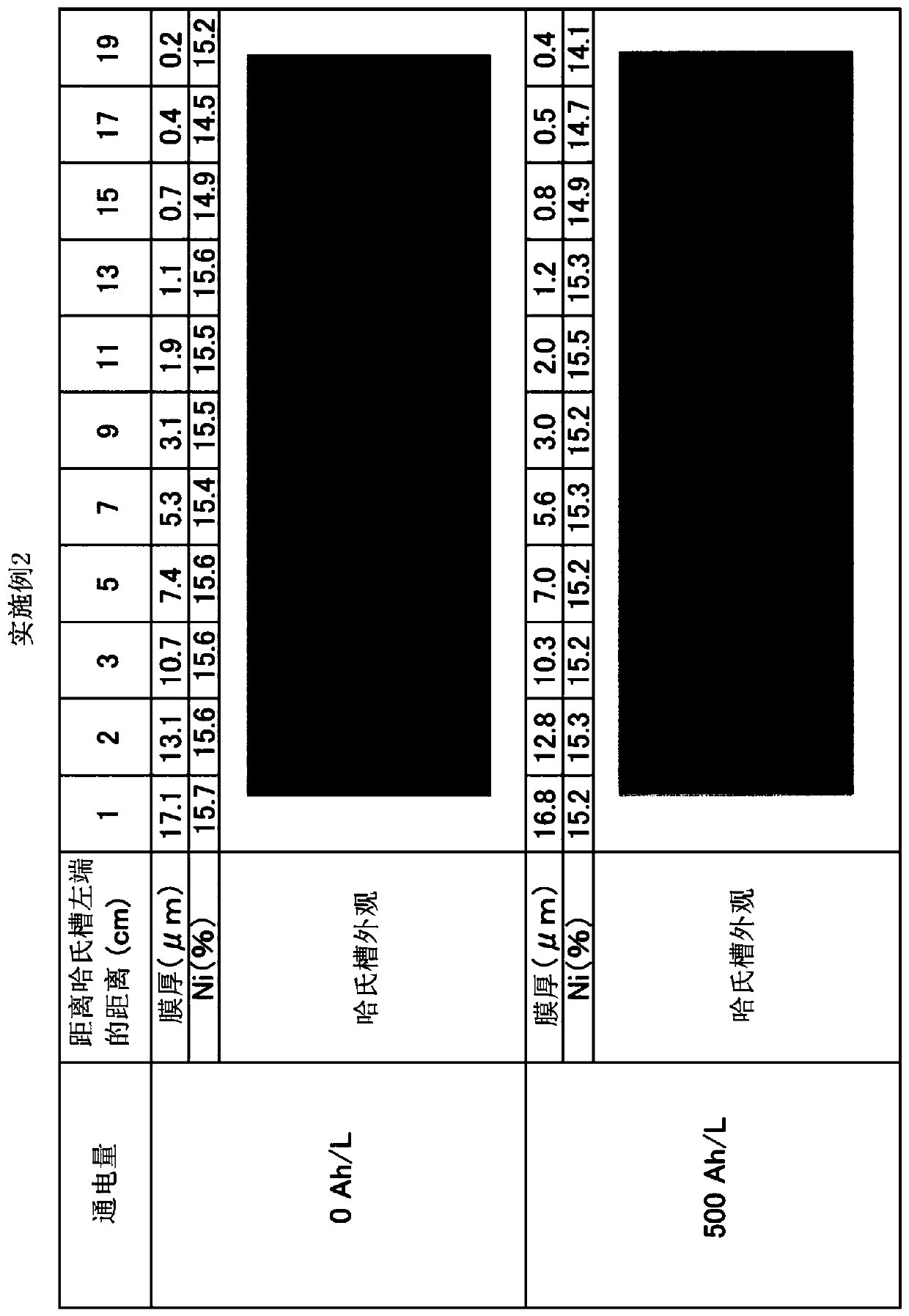
Embodiment 2
[0091] An anode plate (surface roughness Ra: 4 μm, 64×64×2 mm) coated with tantalum oxide on Fe with a thickness of 0.5 to 0.8 μm was used, and an alkaline zinc-nickel alloy plating bath (500 mL ), using 500Ah / L energization to implement zinc-nickel alloy plating. The pore diameter in the coating film was 0.1 to 1 μm, and the dipping out of the plating bath was 2 mL / Ah. The cathode current density is 4A / dm 2 , the anode current density is 9.8A / dm 2 , the plating bath temperature is 25°C. The bath was cooled and maintained at 25°C. An iron plate is used for the cathode. It should be noted that the iron plate of the cathode was replaced every 16Ah / L during energization. The concentration of zinc ions in the plating bath is maintained constant by dipping and dissolving metal zinc. The concentration of nickel ions in the plating bath was kept constant by supplying nickel replenisher IZ-250YNi (manufactured by DIPSOL). The sodium hydroxide concentration of the plating bath i...
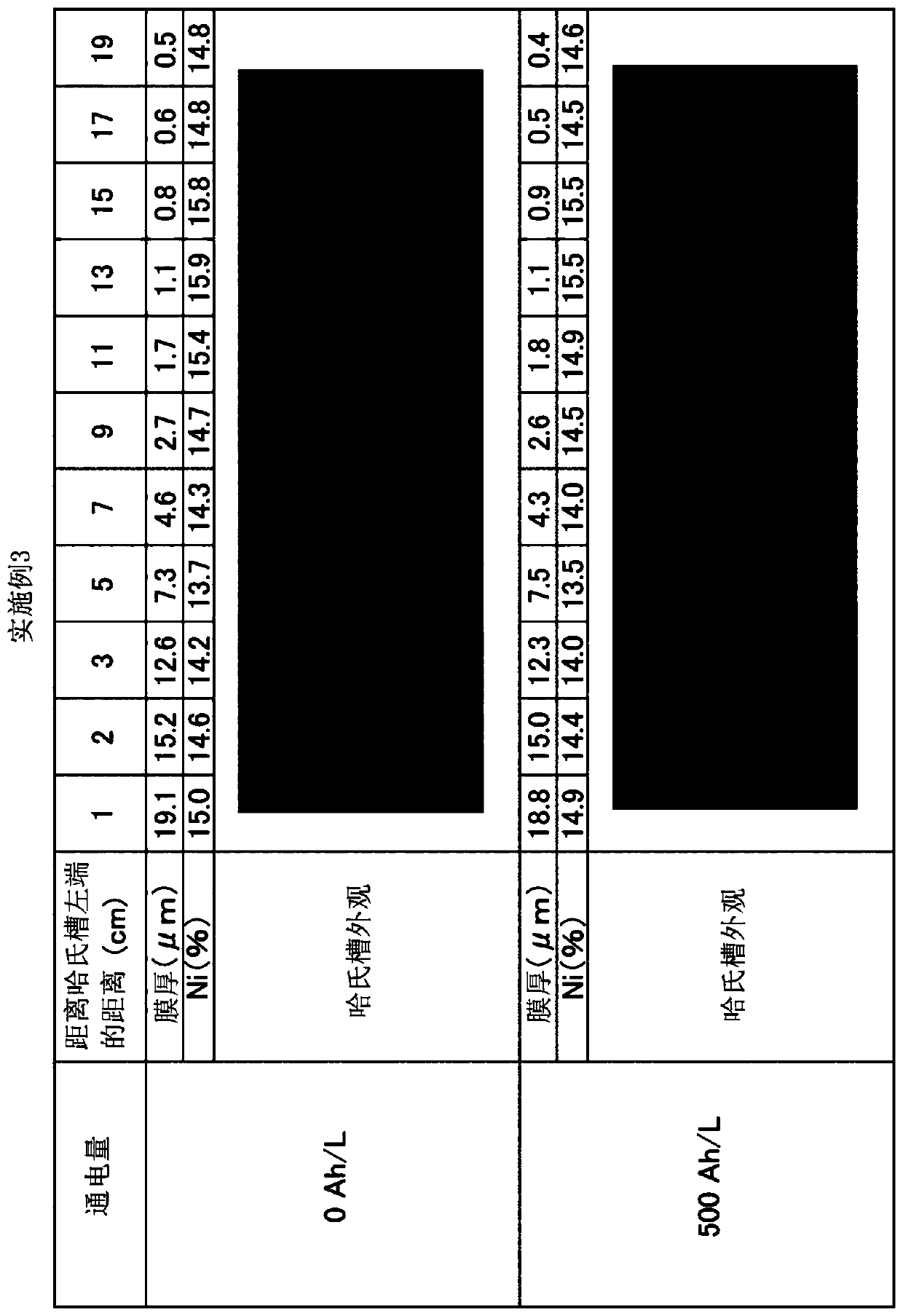
Embodiment 3
[0099] An anode plate (surface roughness Ra: 4 μm, 64×64×2 mm) coated with tantalum oxide on Ni with a thickness of 0.5 to 0.8 μm was used, and an alkaline zinc-nickel alloy plating bath (500 mL ), using 500Ah / L energization to implement zinc-nickel alloy plating. The pore diameter in the coating film was 0.1 to 1 μm, and the dipping out of the plating bath was 2 mL / Ah. The cathode current density is 2A / dm 2 , the anode current density is 4.9A / dm 2 , the plating bath temperature is 25°C. The bath was cooled and maintained at 25°C. An iron plate is used for the cathode. It should be noted that the iron plate of the cathode was replaced every 16Ah / L during energization. The concentration of zinc ions in the plating bath is maintained constant by dipping and dissolving metal zinc. The concentration of nickel ions in the plating bath was kept constant by supplying nickel replenisher IZ-250YNi (manufactured by DIPSOL). The sodium hydroxide concentration of the plating bath i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com