Primary hepatocyte cryopreservation solution, hepatocyte cryopreservation method and hepatocyte resuscitation method
A technology of primary hepatocytes and cryopreservation solution, applied in the field of primary hepatocyte cryopreservation solution, can solve the problems of poor effect of cryopreservation of liver cells, difficult for cells to adhere to the wall, and low cell viability, so as to protect the liver cell membrane and enhance the vitality. The effect of high adherence ability and cell viability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082]The primary hepatocyte cryopreservation solution of this embodiment is composed of the following components in volume percentage: 45% solution A, 10% fetal bovine serum, 10% dimethyl sulfoxide and 35% water, wherein, The solution A is composed of the following components: 100mg / mL D-glucose, 130mg / mL hydroxyethyl starch, 70mg / mL lactobionic acid, 60mg / mL polyvinylpyrrolidone, 30mg / mL pentahydrate D-raffinose, 15mg / mL potassium hydroxide, 6mg / mL potassium dihydrogen phosphate, 4mg / mL magnesium sulfate heptahydrate, 2mg / mL adenosine, 3mg / mL reduced glutathione, 0.2mg / mL allopurinol, 7×10 -3 mg / mL tauroursodeoxycholic acid and 1×10 -3 mg / mL- diammonium glycyrrhizinate, adjust the pH value to 7.4, and store at 4°C.
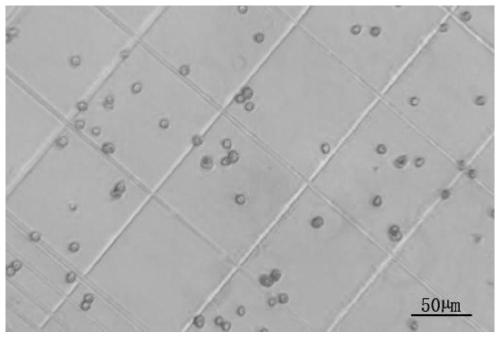
[0083] A method for cryopreserving hepatocytes, comprising: adding the above-mentioned primary hepatocyte cryopreservation solution to hepatocytes that have undergone conventional treatment, mixing evenly, and then cryopreserving. Specifically: add the above-m...
Embodiment 2
[0096] The primary hepatocyte cryopreservation solution of the present embodiment is composed of the following components in volume percentage: 45% solution A, 45% fetal calf serum and 10% dimethyl sulfoxide, wherein the solution A consists of The following components are composed: 120mg / mL D-glucose, 111mg / mL hydroxyethyl starch, 79.6mg / mL lactobionic acid, 51mg / mL polyvinylpyrrolidone, 39.6mg / mL pentahydrate D-raffinose, 12.5mg / mL mL potassium hydroxide, 7.56mg / mL potassium dihydrogen phosphate, 2.73mg / mL magnesium sulfate heptahydrate, 2.98mg / mL adenosine, 2mg / mL reduced glutathione, 0.3mg / mL allopurinol, 6× 10 -3 mg / mL tauroursodeoxycholic acid and 1 mg / mL diammonium glycyrrhizinate, adjust the pH value to 7.4, and store at 4°C.
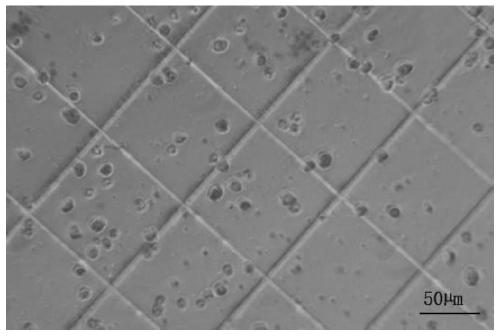
[0097] A method for cryopreserving hepatocytes, comprising: adding the above-mentioned primary hepatocyte cryopreservation solution to hepatocytes that have undergone conventional treatment, mixing evenly, and then cryopreserving. Specifically:...
Embodiment 3
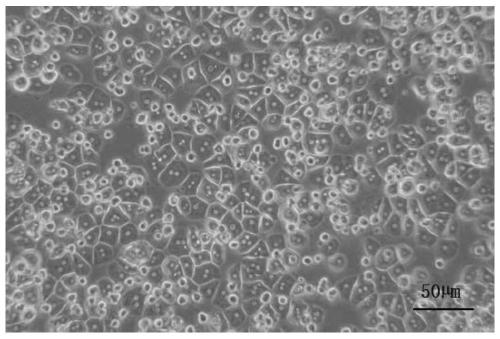
[0110] The primary hepatocyte cryopreservation solution of this embodiment is composed of the following components in volume percentage: 45% of solution A, 25% of human serum albumin, 10% of dimethyl sulfoxide and 20% of water, wherein , the solution A is composed of the following components: 140mg / mL D-glucose, 90mg / mL hydroxyethyl starch, 90mg / mL lactobionic acid, 40mg / mL polyvinylpyrrolidone, 50mg / mL pentahydrate D-raffinose , 10mg / mL potassium hydroxide, 9mg / mL potassium dihydrogen phosphate, 2mg / mL magnesium sulfate heptahydrate, 4mg / mL adenosine, 1mg / mL reduced glutathione, 0.4mg / mL allopurinol, 5× 10 -3 mg / mL tauroursodeoxycholic acid and 10 mg / mL diammonium glycyrrhizinate. Human serum albumin can be purchased commercially, such as purchased from Shenzhen Weiguang Biological Products Co., Ltd., National Drug Approval S20033032, wherein the protein concentration is 20%. When used in this embodiment, use ultrapure water (ddH 2 O) Dilute to 3v / v%. Adjust the pH to 7.4 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com