Natural killer cell expressing anti-cotinine chimeric antigen receptor
A technology of natural killer cells and chimeric antigen receptors, applied in the direction of receptors/cell surface antigens/cell surface determinants, antibodies, animal cells, etc., to achieve high-efficiency anti-cancer effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1. Carrier Skeleton
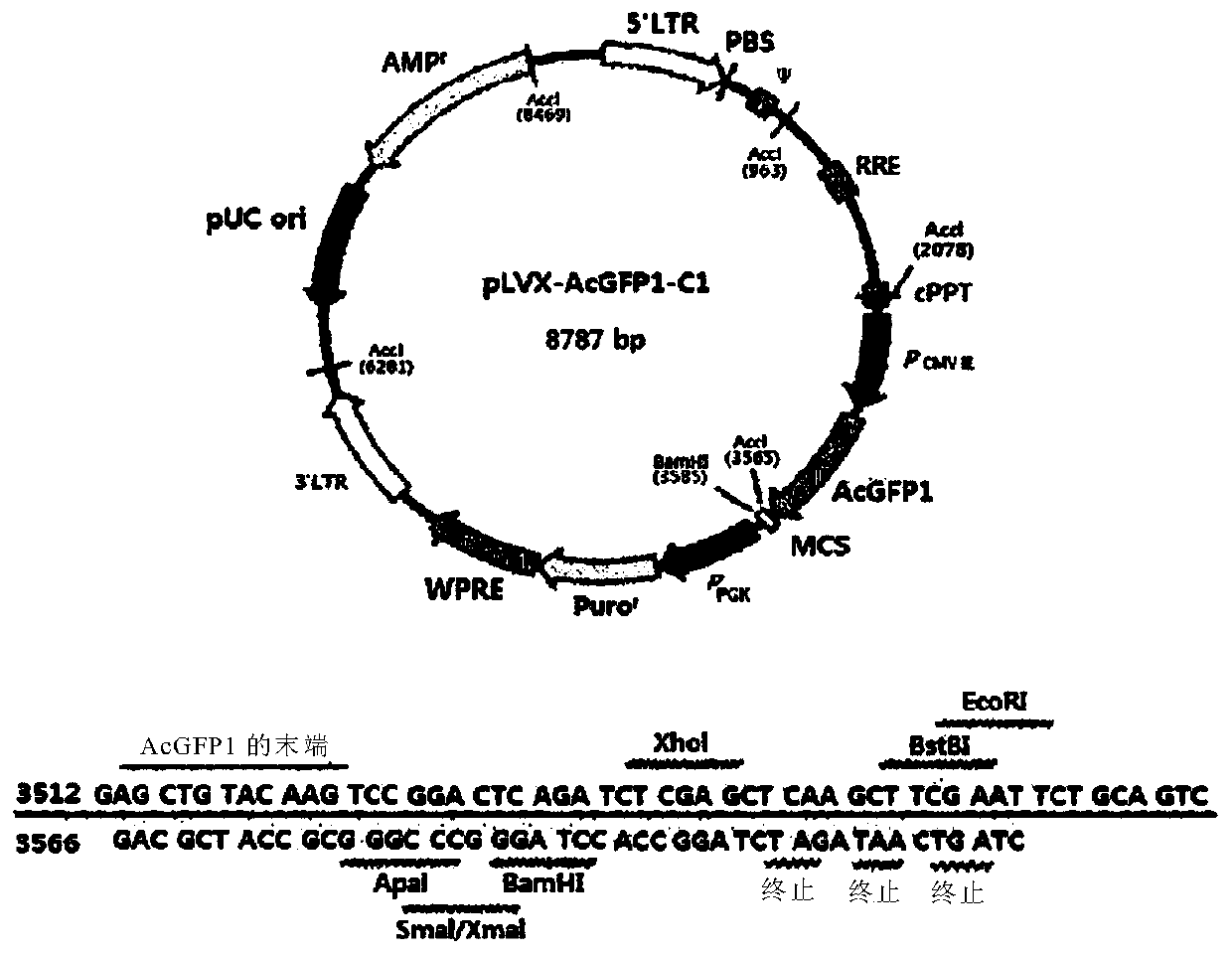
[0095] As the vector used in the present invention, a lentiviral vector (Clontech, 632155) was used. Specifically, using figure 1 pLVX-AcGFP-C1 as indicated. The Kozak sequence (CTCGAG; nucleotides 2801-2806) and AcGFP1 (Aequorea coerulescens green fluorescent protein; nucleotides 2807-3604) were deleted for use in the experiment, then XhoI was used as a restriction enzyme. The specific related sequences are shown in Figure 4 .
Embodiment 2
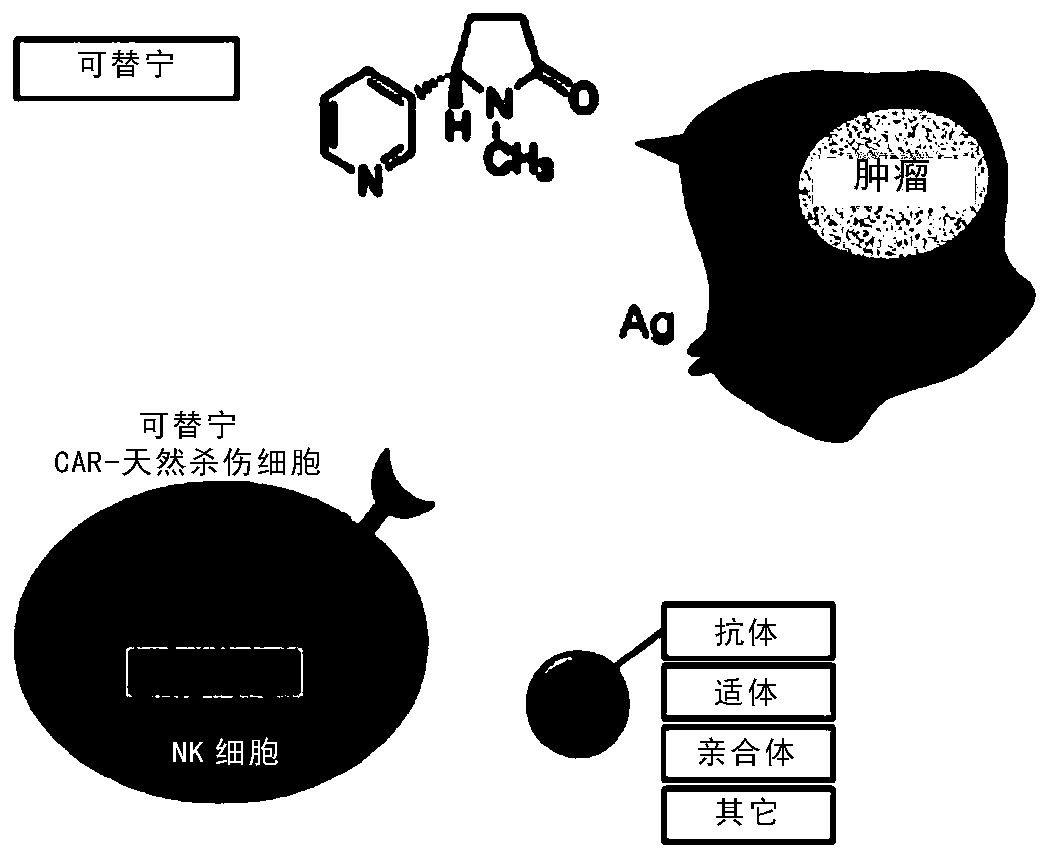
[0096] Example 2. Preparation of Target Antigen and Cotinine Conjugate
[0097] Cotinine (trans-4-cotinine carboxylic acid) is a small molecular substance used as a target antigen, and its chemical structure is shown in Formula 1 below. Cotinine from Sigma-Aldrich was used.
[0098] [Formula 1]
[0099]
[0100] In addition, a conjugate in which cotinine is fused to a binding substance is prepared by the following method.
[0101] Specifically, for the HER2-cotinine conjugate, a conjugate of cotinine and anti-HER2 antibody was prepared using Trastuzumab (Genentech, USA) as an anti-HER2 antibody. Here, the conjugate was coupled by the 1-ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) coupling method. First, an anti-HER2 antibody was prepared by dissolving in PBS at a concentration of 25 μM. On the other hand, prepare trans-4-cotinine carboxylic acid by dissolving in 1 ml of MES buffer [0.1M 2-[morpholino]ethanesulfonic acid (MES) and 0.5M NaCl, pH 6.0] (Sigma-Aldrich ...
Embodiment 3
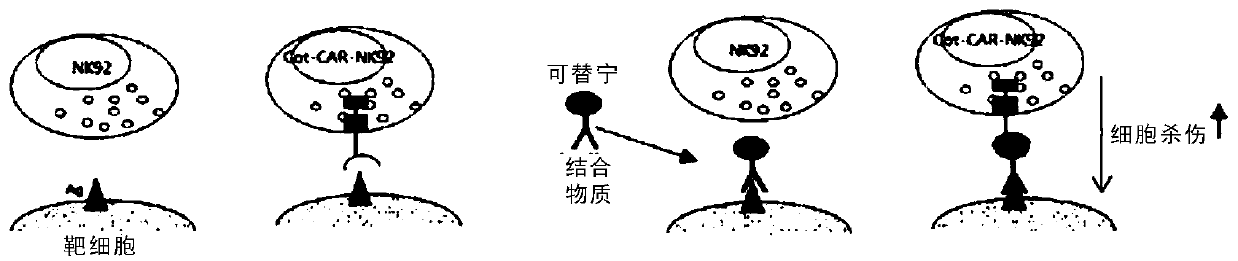
[0104] Example 3. Anti-cotinine chimeric antigen receptor
[0105] A plasmid containing a nucleic acid encoding each domain of the chimeric antigen receptor of the present invention that specifically binds cotinine was prepared by the following method.
[0107] Based on the human T cell surface glycoprotein CD8α chain (GenBank: AK300089.1), two types of primers (forward primer: SEQ ID NO: 18, reverse primer: SEQ ID NO: 19) Perform polymerase chain reaction followed by cloning.
[0108] (2) Target-specific recognition domain-scFv
[0109]As an antigen-binding domain capable of specifically binding cotinine, it is desired to obtain an anti-cotinine chimeric antibody or an antibody fragment thereof. For the sequence of ScFv, refer to information related to ScFv in Korean Patent No. 10-1648960. Specifically, the antigen-binding domain includes the nucleotide sequence shown in SEQ ID NO: 17, and is constructed as VH-linker-VL.
[0110] (3) Junction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com