Edaravone sodium chloride injection and preparation technology
A technology of edaravone sodium chloride and preparation technology, applied in the field of injection and preparation technology, can solve problems such as loss of drug efficacy, increase in filtration cost, etc., and achieves reduction of total amount of bacteria, reduced time, good sterilization and sterilization effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
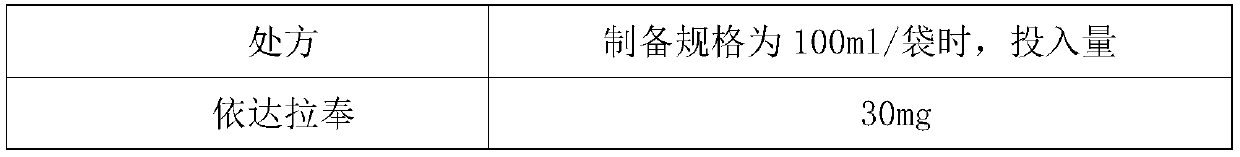
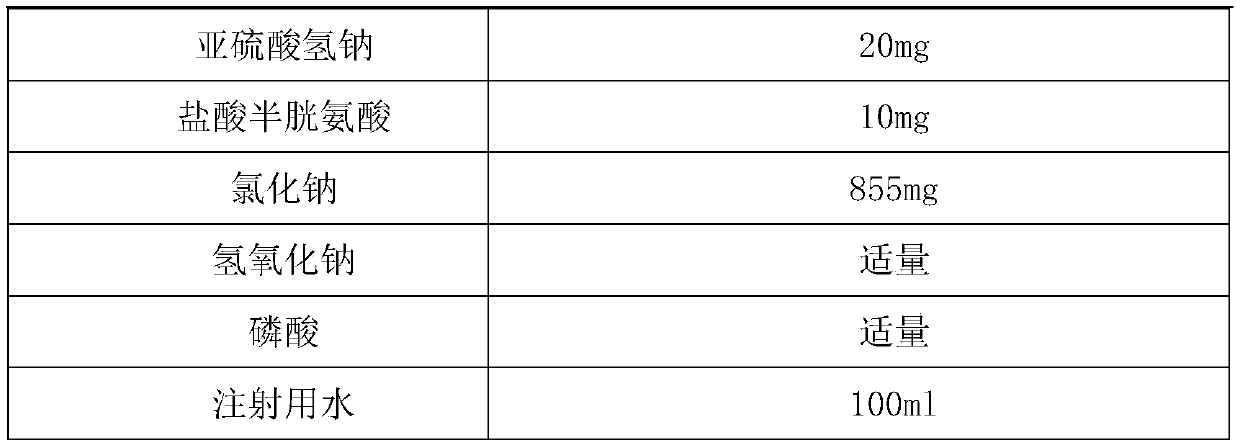
[0060] Prepare the Edaravone Sodium Chloride Injection of 100ml infusion bag. A total of 16000 bags of liquid medicine were prepared.
[0061] The ratio is as follows:
[0062]
[0063]
Embodiment 2
[0065] 1. Add about 80% of the prescribed amount of water for injection into the liquid preparation tank, set the water temperature to 46°C, fill with nitrogen for 25 minutes, monitor the dissolved oxygen and residual oxygen, so that the dissolved oxygen is less than 2mg / L, and the residual oxygen is less than 3%.
[0066] 2. Add the prescribed amount of cysteine hydrochloride, sodium chloride, and sodium bisulfite in turn, and stir for 10 minutes to dissolve completely.
[0067] 3. Then add 0.01% medicinal activated carbon moistened with water for injection, and keep stirring at 46°C for 15 minutes.
[0068] 4. Pass the solution in the concentrated preparation tank through a titanium rod filter for decarbonization and filtration for 60 minutes, remove the titanium rod filter, connect the pipeline, pre-dissolve the prescribed amount of Edaravone with water for injection, and then add it to the liquid preparation tank , The water temperature in the liquid mixing tank is 46°C,...
Embodiment 3
[0080] 1. Add about 80% of the prescribed amount of water for injection into the liquid preparation tank, set the water temperature to 50°C, fill with nitrogen for 30 minutes, monitor the dissolved oxygen and residual oxygen, so that the dissolved oxygen is less than 2mg / L, and the residual oxygen is less than 3%.
[0081] 2. Add the prescribed amount of cysteine hydrochloride, sodium chloride, and sodium bisulfite in turn, and stir for 10 minutes to dissolve completely.
[0082] 3. Then add medicinal activated carbon with a water content of 8% and a concentrated volume of 0.01% moistened with water for injection, and keep stirring at 50°C for 15 minutes.
[0083] 4. Pass the solution in the concentrated preparation tank through a titanium rod filter for decarbonization and filtration for 60 minutes, remove the titanium rod filter, connect the pipeline, pre-dissolve the prescribed amount of Edaravone with water for injection, and then add it to the liquid preparation tank , ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com