Novel melittin variant and application thereof
A kind of melittin, a new type of technology, applied in the new variant of melittin and its application field, can solve problems such as unsatisfactory antibacterial ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
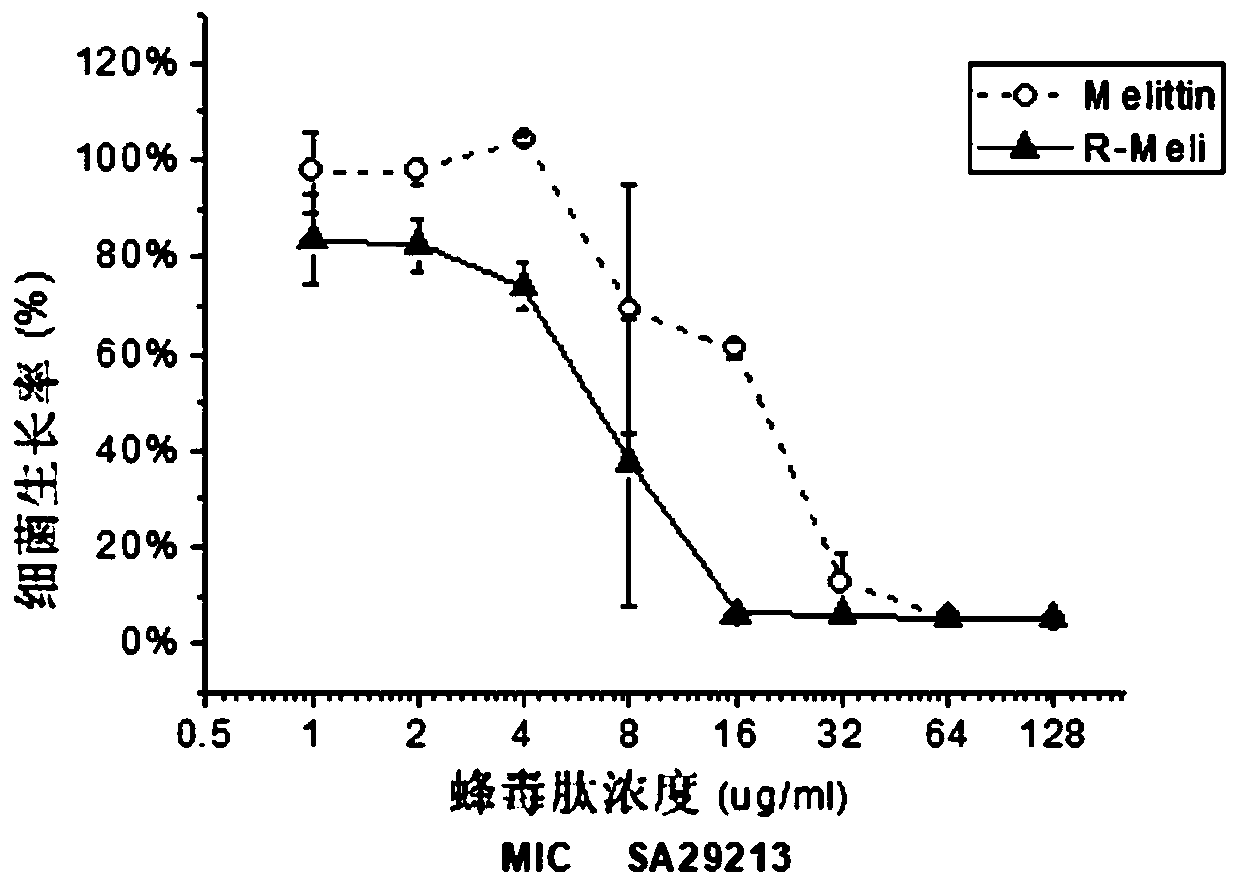
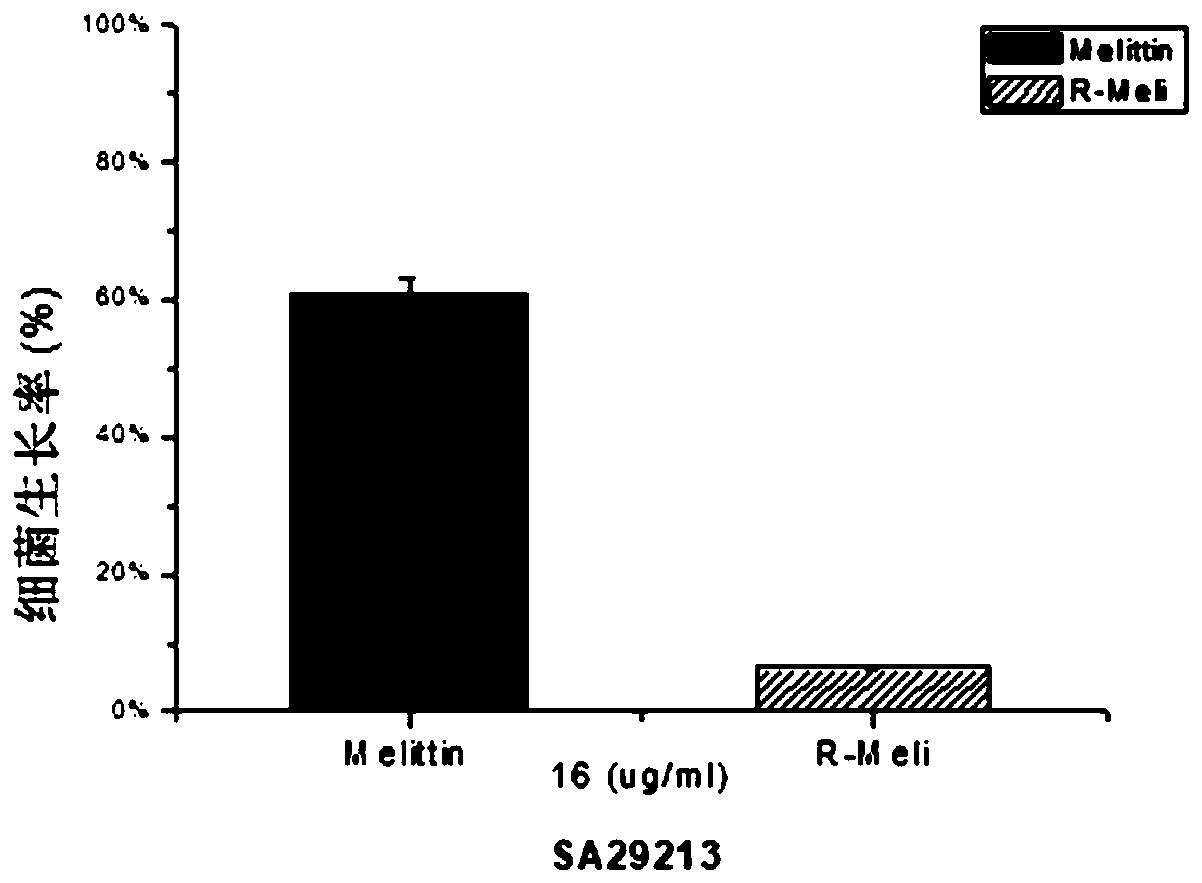
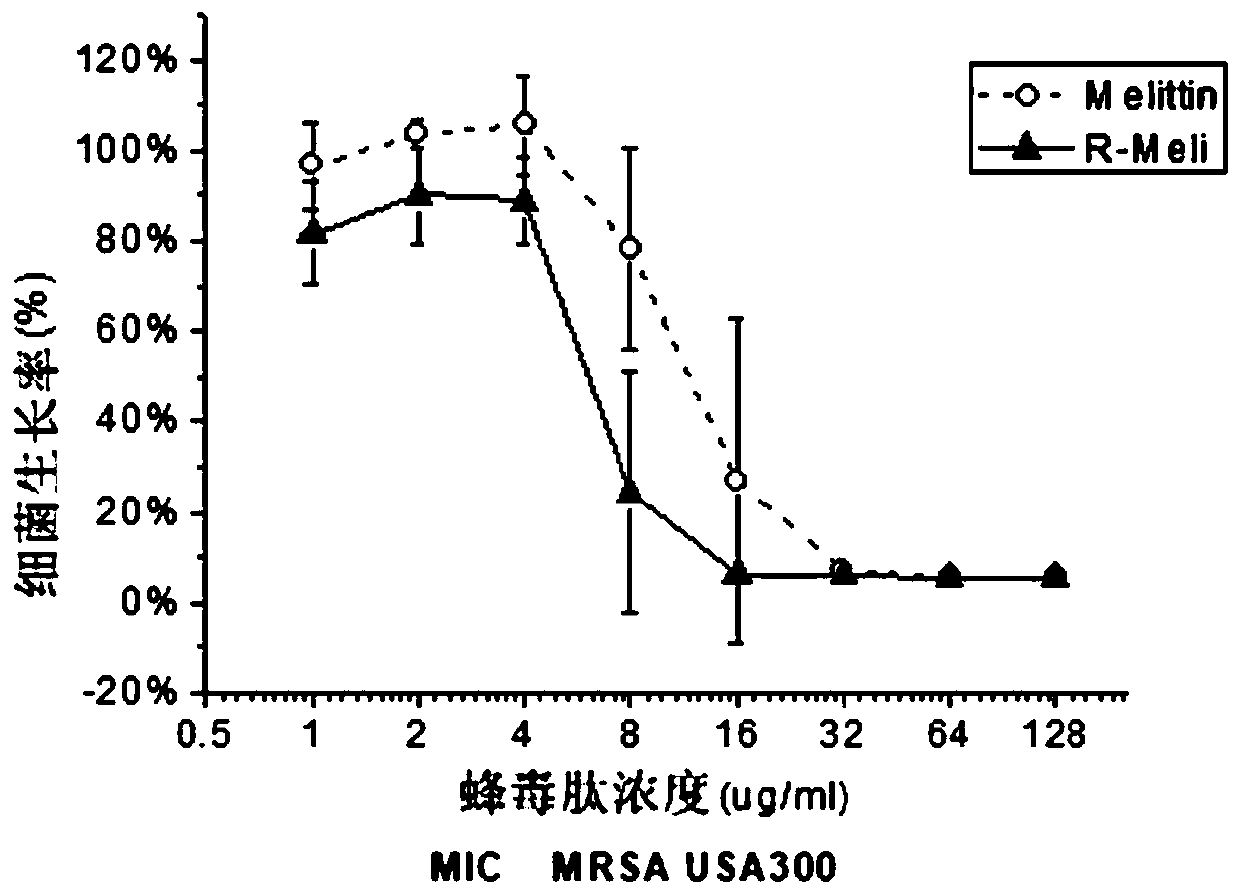
[0039] Embodiment 1 polypeptide design and antibacterial test
[0040] 1. Design of the polypeptide Based on the melittin polypeptide sequence and synthesis, the melittin-R sequence (R-Meli) was designed:
[0041] Melittin sequence: GIGAVLKVLTTGLPALISWIKRKRQQ-NH2
[0042] Melittin-R (R-Meli) sequence: GIGAVLRVLTTGLPALISWIRRRRQQ-NH2
[0043] 2. Antibacterial test
[0044] The minimum inhibitory concentration (MIC) of melittin-R was tested using the broth dilution method.
[0045] 2.1 MIC plate preparation
[0046] The sample solution with a concentration of 256 mg / ml was respectively injected into the first row of wells of a 96-well polystyrene plate, 100 ul per well, and each sample was repeated 3 times. Add 50ul LB liquid medium to each hole in the back row, use a row gun to draw 50ul sample solution from the first row, blow and mix it with the second row, and then draw 50ul and add it to the third plate and mix it by blowing and blowing, and then perform two-fold dilutio...
Embodiment 2
[0054] Embodiment 2 erythrocyte hemolysis experiment
[0055] 1. Experimental reagents:
[0056] TBS buffer: 605mg Tris (final concentration 10mM TRIS), 4.4gNacl (150mM NaCl), 500mlddH 2 O, pH=7.2;
[0057] Red blood cell lysate: 0.2% Triton X-100 diluted with TBS buffer.
[0058] 2. Experimental steps:
[0059] 1) The blood sample was taken from normal human blood, centrifuged at 1000g for 5min, removed the supernatant, added an equal amount of TBS buffer, mixed gently, centrifuged again at 1000g for 5min, and repeated 3-5 times until the supernatant was clear.
[0060] 2) Collect the resulting precipitate, dilute it in TBS buffer at 1:50, and gently mix it by inverting back and forth to obtain a red blood cell suspension.
[0061] 3) Adding samples to the 96-well plate: use the method of sample injection and dilution with a row gun to form a total of 8 concentration gradients of 128, 64, 32, 16, 8, 4, 2, and 1ug / ml in each sample solution of the experimental group, 100ul...
Embodiment 3
[0067] Example 3 Toxicity test of melittin-R to tumor cells
[0068] 1. Experimental reagents:
[0069] MTS cytotoxic staining reagent was purchased from Promega Company. DMEM high-glucose cell culture medium, 10% fetal bovine serum + 90% DMEM, was purchased from the Cell Resource Center of the Chinese Academy of Medical Sciences.
[0070] Tumor cells: SW620 human colon cancer cells.
[0071] 2. Experimental steps:
[0072] 1) Resuscitate SW620 cells and passage them for more than 2 generations. After the cells grow stably, take a plate of well-grown cells to digest and count the plate. Adjust the concentration of the cell suspension according to the statistical value and add it to a 96-well plate. Add cells as a blank group, and the number of cells per well is about 20,000. Treat overnight at 37°C in a 5% carbon dioxide incubator. After 12 hours, the cells complete attachment.
[0073] 2) After the cells adhere to the wall, carefully suck out the old medium in the 96-well...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com