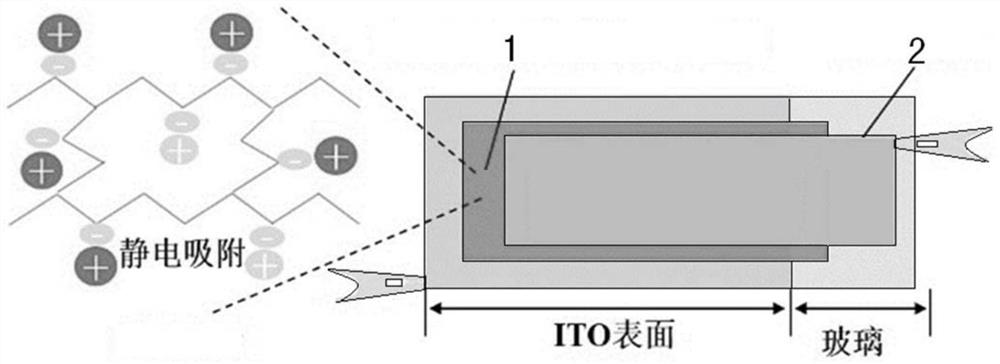
Ion-conductive electrochemiluminescence hydrogel for light-emitting display and preparation method thereof
An ion-conducting and electrochemical technology, applied in chemical instruments and methods, organic chemistry, luminescent materials, etc., can solve problems such as inability to effectively fix the medium, easy diffusion and loss of the medium, affecting display performance, etc., and is conducive to mass production, The effect of low initiation voltage and low fabrication cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1) Use a balance to weigh 0.1564g of AM, 0.02g of MBAA and 0.0362g of NaAA, dissolve the weighed solid powder (AM, MBAA, NaAA) in 500μL of deionized water in sequence, shake and mix at room temperature for 2 to 3 minutes to obtain Colorless clear solution.
[0042] 2) Weigh 0.0037g of Ru(bpy) with a balance 3 Cl 2 , 0.0072g of TPA and 0.0292g of NaCl, the weighed material (Ru(bpy) 3 Cl 2 , TPA, NaCl) were sequentially added to the colorless clear solution of the above 1) and shaken and mixed at room temperature for 2 to 3 minutes to obtain a red transparent solution.
[0043] 3) Add 0.0009 g of APS and 0.0078 g of TEMED to the red transparent solution in 2), shake and mix at room temperature for 2 to 3 minutes to fully dissolve and add water to a total volume of 1 mL to obtain the final mixed solution.
[0044] 4) After the final mixed solution was allowed to stand at room temperature for 60 minutes, the target hydrogel ( figure 2 ).
[0045] The target hydrogel ...
Embodiment 2
[0050] 1) Weigh 0.1564g of AM, 0.02g of MBAA and 0.0362g of NaAA with a balance, dissolve the above-mentioned solid powders (AM, MBAA, NaAA) in sequence in 500μL of deionized water, shake and mix at room temperature for 2 to 3 minutes A colorless clear solution was obtained.
[0051] 2) Weigh 0.0008g of Ru(bpy) with a balance 3 Cl 2 , 0.0014g of TPA and 0.0292g of NaCl, the weighed material (Ru(bpy) 3 Cl 2 , TPA, NaCl) were sequentially added to the colorless clear solution of the above 1) and shaken and mixed at room temperature for 2 to 3 minutes to obtain a red transparent solution.
[0052] 3) Add 0.0009 g of APS and 0.0078 g of TEMED to the red transparent solution in 2), shake and mix at room temperature for 2 to 3 minutes to fully dissolve and add water to a total volume of 1 mL to obtain the final mixed solution.
[0053] 4) The final mixed solution was left to stand at room temperature for 60 minutes to obtain a red transparent hydrogel.
[0054] After identifica...
Embodiment 3
[0057] 1) Weigh 0.1564g of AM, 0.02g of MBAA and 0.0362g of NaAA with a balance, dissolve the above-mentioned solid powders (AM, MBAA, NaAA) in sequence in 500μL of deionized water, shake and mix at room temperature for 2 to 3 minutes A colorless clear solution was obtained.
[0058] 2) Weigh 0.0037g of Ru(bpy) with a balance 3 Cl 2 , 0.0072g of TPA and 0.0058g of NaCl, the weighed material (Ru(bpy) 3 Cl 2 , TPA, NaCl) were sequentially added to the colorless clear solution of the above 1) and shaken and mixed at room temperature for 2 to 3 minutes to obtain a red transparent solution.
[0059] 3) Add 0.0009 g of APS and 0.0078 g of TEMED to the red transparent solution in 2), shake and mix at room temperature for 2 to 3 minutes to fully dissolve and add water to a total volume of 1 mL to obtain the final mixed solution.
[0060] 4) The final mixed solution was heated in an oven at 60° C. for 30 minutes to obtain a red transparent hydrogel.
[0061] After identification, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com