Method for extracting soy isoflavone aglycone from soybean milk water
A technology for isoflavone aglycone and soybean milk, applied in the field of extracting soybean isoflavone aglycone, can solve the problems of low purity and complexity of consumption of soybean isoflavone glycoside and soybean isoflavone aglycone, and achieve improved purity, low purity and large investment. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Take the alkaline extraction and acid precipitation process to produce 4L of soy milk water discharged from the soybean protein, and naturally settle for 4h to obtain 1000 mL of yellow liquid in the lower layer (the supernatant is colorless). Add 15g of activated clay to the liquid, magnetically stir for 0.5h, and filter to obtain activated clay residue. After natural drying or drying (<140℃), add 30mL of ethyl acetate, magnetically stir for 60min, and centrifuge at 5000r / min for 12min. Take the ethyl acetate supernatant and spin-evaporate to dryness to obtain 12.5 mg of product, and the yield of soy isoflavone aglycone is 85%.
[0036] To analyze the obtained products, the method is as follows:
[0037] 1. Prepare 6 standard solutions of daidzein, daidzein, genistein, genistein, glycitein, and glycitin into 6 standard solutions of 1 mg / mL.
[0038] 2. The product prepared in this embodiment was dissolved in 10 mL of ethyl acetate as a sample liquid.
[0039] 3. Using the acti...
Embodiment 2
[0046] Take the alkaline extraction and acid precipitation process to produce 4L of soy milk water discharged from the soybean protein, and naturally settle for 5 hours to obtain 1000 mL of yellow liquid in the lower layer (the supernatant is colorless). Add 10g of activated clay to the liquid, magnetically stir for 1.5h, and filter to obtain activated clay residue. After natural drying or drying (<140°C), add 20mL of ethyl acetate, magnetically stir for 30min to extract, and centrifuge at 6000r / min for 15min. Take the ethyl acetate supernatant and spin-evaporate to dryness to obtain 12 mg of the product. The yield of soybean isoflavone aglycone is 85%.
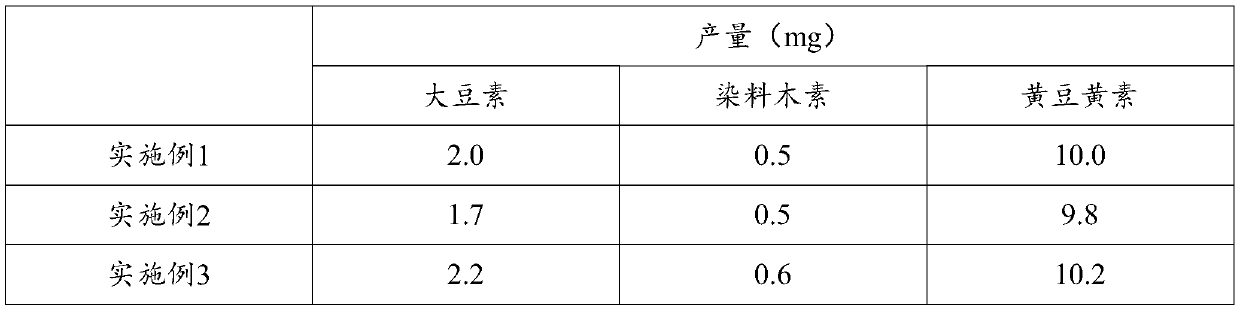
[0047] After testing, the detection method is as in Example 1. The 12 mg product contains only daidzein, genistein, and glycitein, and no soy isoflavone glycosides. Among them, daidzein is 1.7 mg, genistein is 0.5 mg, and soy yellow Vegetarian 9.8mg.
Embodiment 3
[0049] Take the alkaline extraction and acid precipitation process to produce 4L of soy milk water discharged from the soybean protein, and naturally settle for 5 hours to obtain 1000 mL of yellow liquid in the lower layer (the supernatant is colorless). Add 12g of activated clay to the liquid, magnetically stir for 1h, and filter to obtain activated clay residue. After natural drying or drying (<140℃), add 40 mL of ethyl acetate, magnetically stir for 45 minutes, and centrifuge at 4000r / min for 10 minutes. The ethyl acetate supernatant was evaporated to dryness to obtain 13 mg of product, and the yield of soybean isoflavones was 83%.
[0050] After testing, the detection method is as in Example 1. The 13 mg product contains only daidzein, genistein, and glycitein, and no soy isoflavone glycosides. Among them, daidzein is 2.2 mg, genistein 0.6 mg, and soybean yellow Vegetarian 10.2mg.
[0051] The yield of soybean isoflavone aglycone prepared in Examples 1 to 3 is shown in Table 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com