Method and kit for rapidly detecting immunosuppressive agent in whole blood and application of kit
An immunosuppressant, fast technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problem that it is difficult to meet the needs of clinical use, cannot detect immunosuppressants at the same time, cannot monitor therapeutic drugs for five immunosuppressants at the same time, etc. problems to meet the clinical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
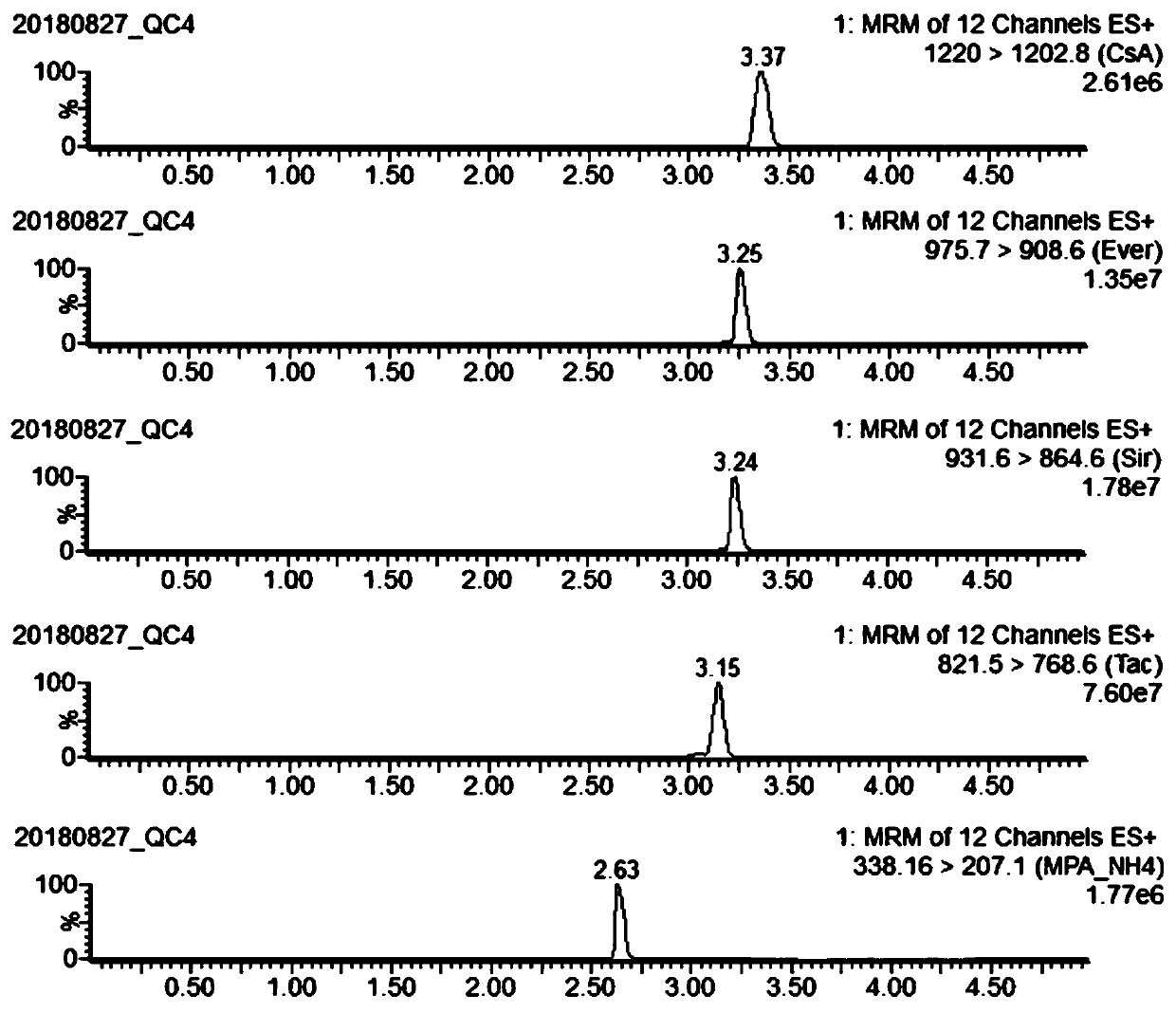
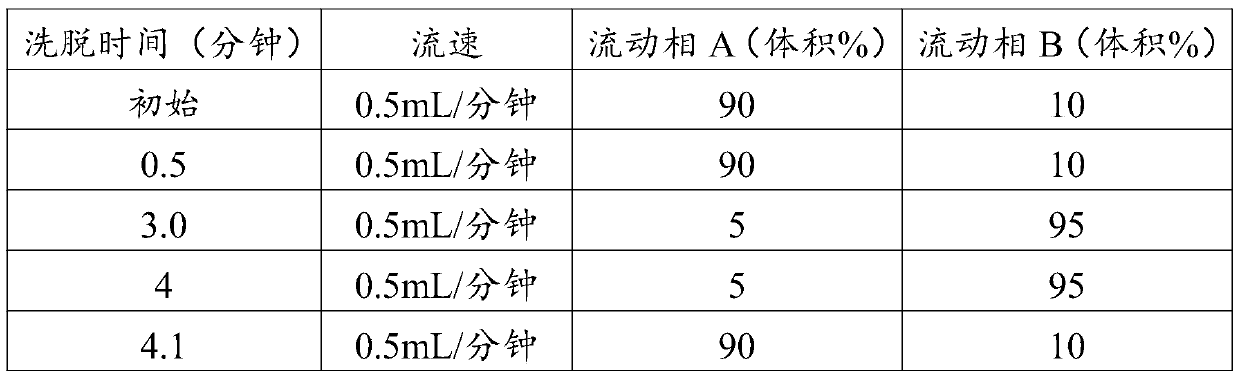
[0049] In this example, the kit used for rapid detection of immunosuppressants in whole blood, the composition of which is shown in Table 2.
[0050] Table 2 Kit Composition
[0051]
[0052] In Table 2, 1 bottle of internal standard dry powder contains 4 kinds of analyte isotope internal standards, namely cyclosporine A isotope internal standard dry powder 200ng, everolimus isotope internal standard dry powder 10ng, sirolimus isotope internal standard dry powder 24ng , Mycophenolic acid isotope internal standard dry powder 200ng.
[0053]In Table 2, one set of standard curve samples includes 200 microliters of whole blood samples without the five immunosuppressants CsA, Tac, Sir, Ever and MPA, that is, blank; and 7 gradient concentrations containing five immunosuppressants at the same time Whole blood samples, namely cal1 to cal7, 100 microliters of each whole blood sample. Among them, the concentration of CsA in the cal1 whole blood sample is 5 ng / mL, the concentration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com