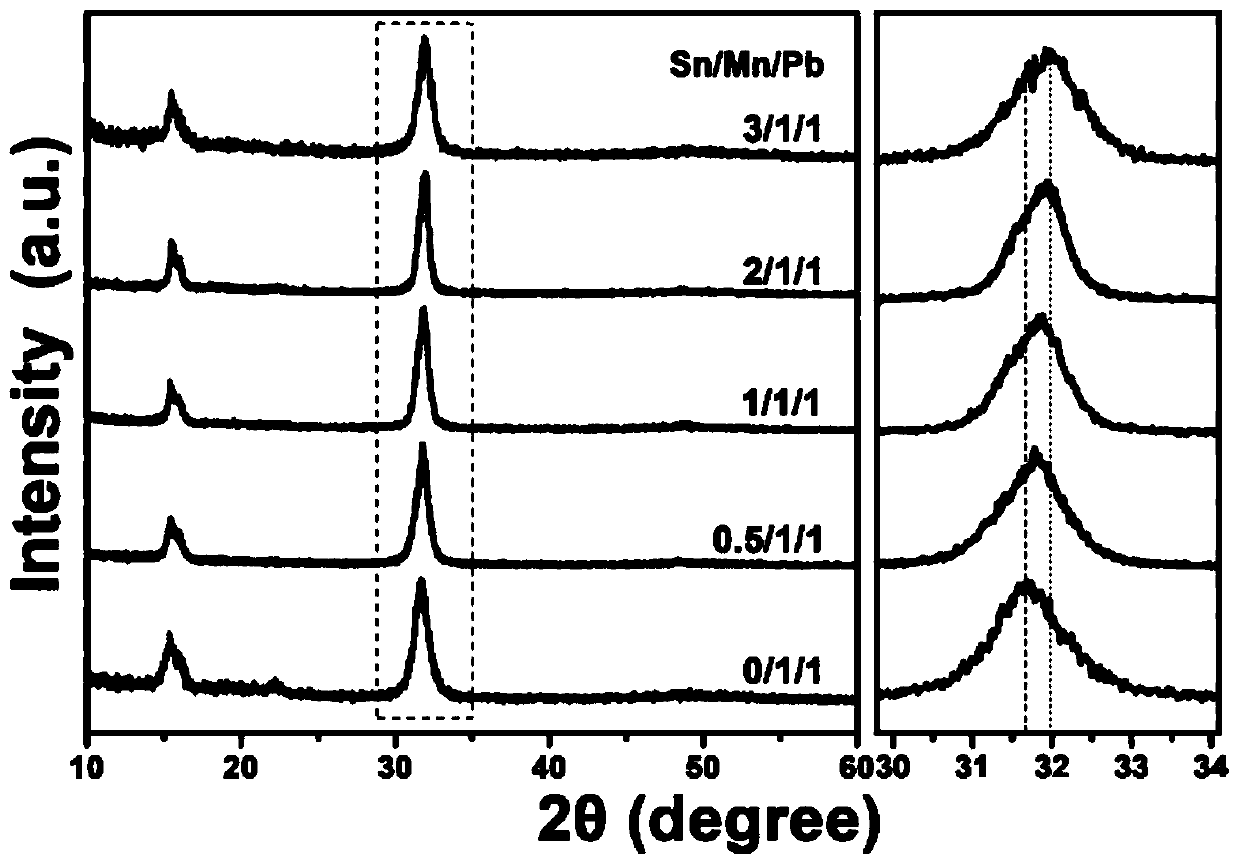
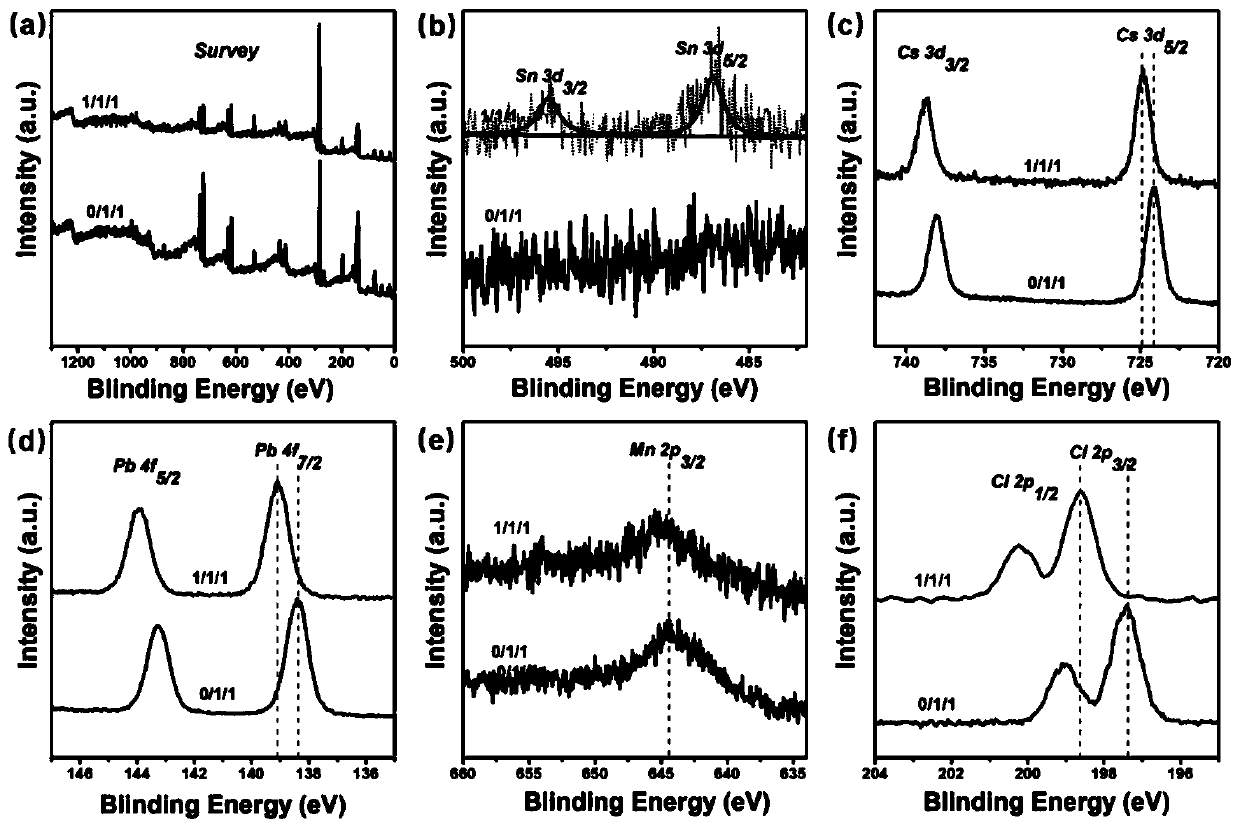
Method for synergistically enhancing ultraviolet radiation stability and optical performance of Mn:CsPbCl3 nanocrystals
A technology of ultraviolet radiation and stability, applied in the fields of nano-optics, chemical instruments and methods, nanotechnology, etc., can solve the problems of reducing the luminescence properties of nanocrystals, complicated preparation methods, increasing coating process steps, etc., and achieve excellent resistance to ultraviolet radiation. Illumination ability, simple process steps, the effect of slowing down the luminous intensity and fluorescence lifetime decay
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] 1) Preparation of cesium oleate solution: put cesium carbonate (0.675mmol) and octadecene (10mL) into a 50mL three-necked flask, and dry at 120°C for 60min under an argon atmosphere; then, inject Oleic acid (1.25mL), the mixed solution was heated to 150°C under the protection of argon, and after keeping for 10 minutes, it was naturally cooled to obtain a cesium oleate solution;
[0036] 2) Preparation of Sn precursor: Put (1mmol) tin salt, octadecene (9mL) and oleylamine (1mL) into a 50mL three-necked flask, and react for 60min at 150°C under an argon atmosphere , naturally cooled to form a Sn precursor;
[0037] 3) Add manganese chloride (0.2mmol), lead chloride (0.2mmol) and Sn precursor (1mL) in step 2) together with octadecene (5mL), oleic acid (1.5mL), oleylamine (1.5mL) and trioctylphosphine (1mL) into a three-necked flask of 50mL specification; after stirring and degassing under vacuum at 110°C for 30min, a transparent solution was formed; in this embodiment, th...
Embodiment 2
[0041] The difference between this example and Example 1 is that in step 3) the Sn precursor (2mL) in manganese chloride (0.2mmol), lead chloride (0.2mmol) and step 2) together with octadecene (5mL), Oleic acid (1.5mL), oleylamine (1.5mL) and trioctylphosphine (1mL) were charged together into a 50mL three-neck flask; after stirring and degassing under vacuum at 110°C for 30min, a transparent solution was formed; this In the embodiment, the molar ratio of Mn / Pb in the Sn precursor, manganese chloride, and lead chloride is 1 / 1; the molar ratio of Sn / Pb is 1 / 1; other steps are the same as in the first embodiment.
Embodiment 3
[0049] 1) Preparation of cesium oleate solution: put cesium carbonate (0.6mmol) and octadecene (9mL) into a 50mL three-necked flask, and dry for 40min under an argon atmosphere at 130°C; then, inject Oleic acid (1mL), the mixed solution was heated to 180°C under the protection of argon, and after keeping for 8 minutes, it was naturally cooled to obtain a cesium oleate solution;
[0050] 2) Preparation of Sn precursor: put (2mmol) tin salt, octadecene (7mL) and oleylamine (3mL) into a 50mL three-necked flask, and react for 50min at 160°C under an argon atmosphere , naturally cooled to form a Sn precursor;
[0051] 3) Manganese chloride (0.2 mmol), lead chloride (0.2 mmol) and Sn precursor (1 mL) in step 2) together with octadecene (8 mL), oleic acid (1 mL), oleylamine (1 mL) and tris Octylphosphine (0.5mL) was packed together in a 50mL three-necked flask; after stirring and degassing under vacuum at 120°C for 25min, a transparent solution was formed; in this example, the Sn pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com