Rosin-based CO2/N2 response type surfactant, and preparation method and application thereof
A surfactant and rosin-based technology, which is applied in the field of rosin-based CO2/N2 responsive surfactants and their preparation, can solve environmental hazards, limit the types of surfactants and other problems, and achieve good responsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
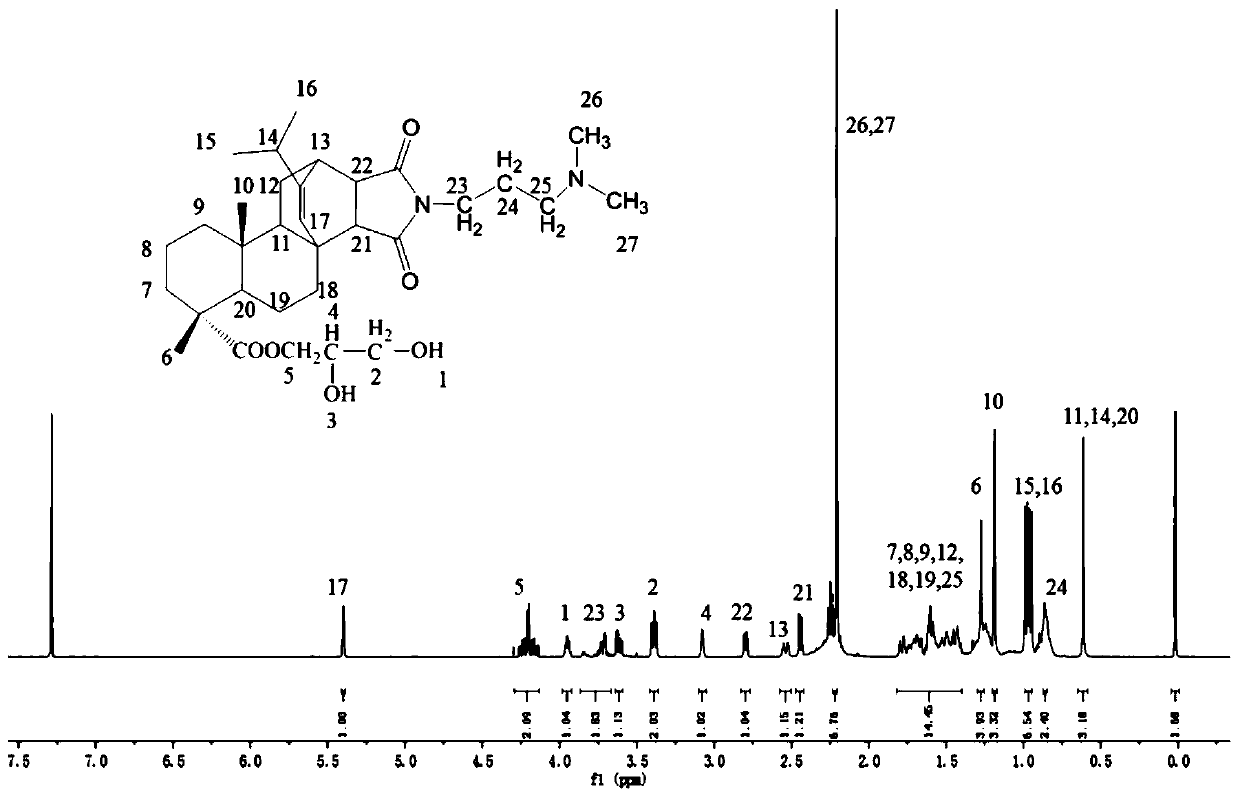
[0032] A rosin-based CO 2 / N 2 Responsive polymerizable surfactant, referred to as MPANG, MPANG's 1 The H NMR spectrum is shown in 1, from figure 1 of 1 The H NMR spectrum shows that the prepared product is the target product. Its structural formula is as follows:
[0033]
Embodiment 2
[0035] A rosin-based CO 2 / N 2 The synthesis of responsive surfactants includes the following steps:
[0036] 100g (0.33mol, the total amount of abietic acid, neoabietic acid and palustric acid is calculated as 80%, 0.265mol) masson pine rosin, 35g (0.357mol) maleic anhydride, 40g acetic acid, react at 140°C for 4h, add after cooling 100g of glacial acetic acid was crystallized by cooling, and suction filtered to obtain maleopimaric acid.
[0037] Add 10g of maleopimaric acid into the reactor and dissolve it in ethanol, and stir at 85°C for 0.5h. Dissolve 2.55g of N,N-dimethyl-1,3-propanediamine in ethanol solvent, add it dropwise into the reactor, continue stirring for 5h after the dropwise addition, after cooling, white crystals precipitate, filter and dry to obtain MPAN
[0038] Add 10g MPAN into the reactor and dissolve it in ethanol. After stirring at 85°C for 0.5h, dissolve 2.36g of glycidyl alcohol in the solvent ethanol and add it into the reactor dropwise. After th...
Embodiment 3
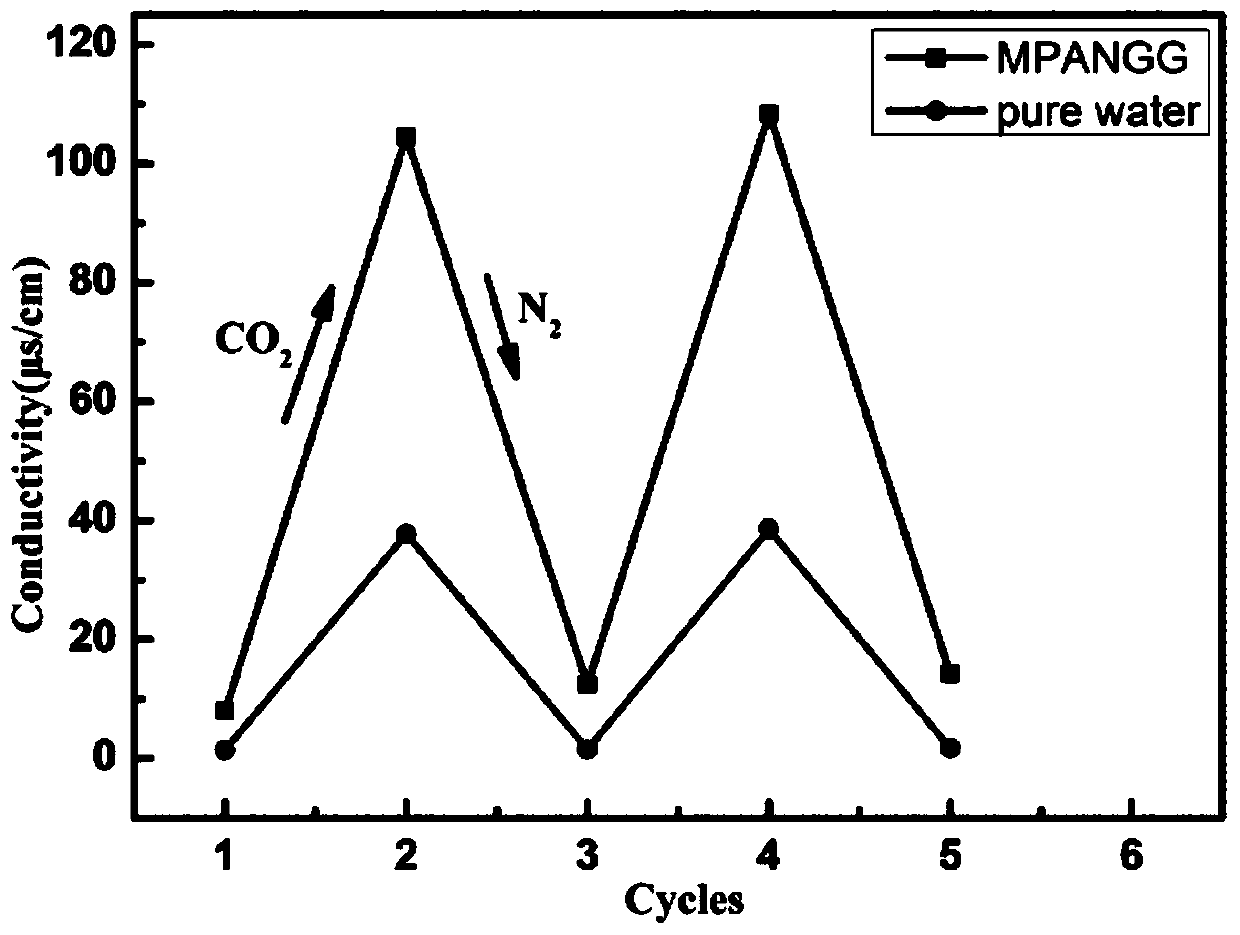
[0041] Judging by the reversible conversion of the conductivity of MPANG solution, the effect of MPANG on CO 2 / N 2 responsiveness. CO 2 Soluble in aqueous solution to form H 2 CO 3 Weak acid solution leads to protonation of the tertiary amine group, generating the charged cationic surfactant MPANGH + The conductivity was raised to about 104.5 μS / cm. When passing N at 60°C 2 drive out CO 2 Afterwards, the weak acidity of the solution disappears, and the charged cationic surfactant deprotonates back to the original tertiary amine group state, so that the conductivity drops back to the initial value. When CO is introduced again 2 Afterwards, the conductivity rises to about 104.5μs / cm again, and this process can be repeated several times to illustrate the effect of MPANG on CO 2 / N 2 It is very responsive. MPANG vs. CO 2 / N 2 The response process is as follows:
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com