A short peptide small molecule self-assembled nanomaterial targeting hypoxic tumors and its preparation method and application
A nanomaterial and self-assembly technology, which is applied in the preparation methods of peptides, anti-tumor drugs, chemical instruments and methods, etc., can solve the problems of lack of hypoxia in cancer treatment, and achieve high biological safety, simple preparation process, and enhanced anti-cancer properties. The effect of tumor efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] This example is used to illustrate the synthesis of isothiocyanate-benzenesulfonamide.
[0107] 4-(2-Aminoethyl)benzenesulfonamide (0.5 mmol) was dissolved in 1 mL of DMF, and triethylamine (3 mmol) was added to the above solution. 1,1'-thiocarbonyldiimidazole (1 mmol) was dissolved in 1.5 mL of DMF at 50°C and added dropwise to the above solution. The solution was stirred continuously for 30 min at room temperature and then added with dd H 2 O quenched. The product was first extracted with ethyl acetate and then purified by silica gel chromatography (ethyl acetate-hexane). The NMR information is as follows: 1 H NMR (400MHz, DMSO-d 6 ) δ7.79(d, J=7.8Hz, 2H), 7.49(d, J=7.8Hz, 2H), 7.35(s, 2H), 3.96(t, 2H), 3.04(t, 2H).
Embodiment 2
[0109] This example is used to illustrate the synthesis of 2-naphthaleneacetic acid-(D)-Phe-(D)-Phe-(D)-Lys-OH.
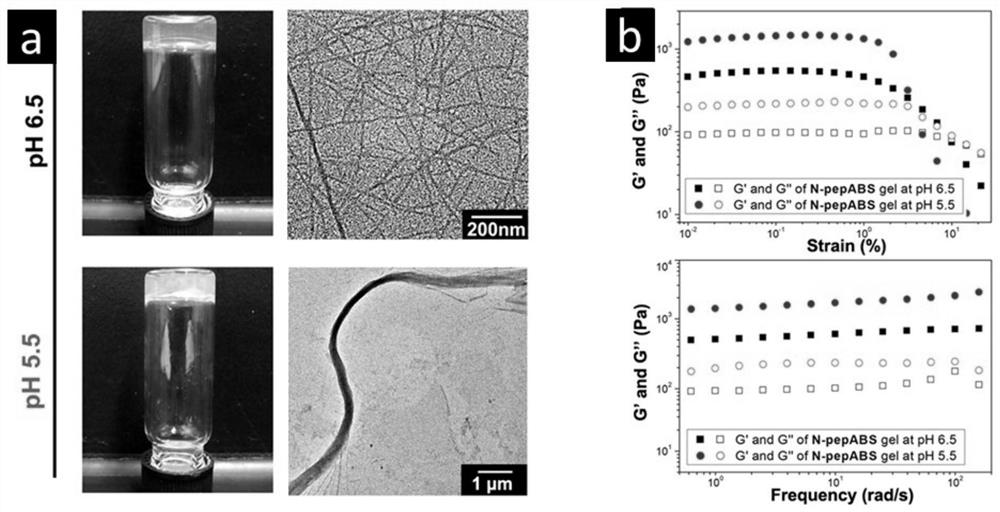
[0110] Short peptide hydrogels were prepared by standard solid-phase peptide synthesis (SPPS), namely 2-chlorotrityl chloride resin (1.10 mmol / g) and with side chain amino groups protected by tert-butoxycarbonyl, and Fmoc protected Various types of D-amino acids in the main chain amino groups are prepared by the reaction. The resin was swelled by bubbling nitrogen in dry dichloromethane (DCM) for 30 minutes, then washed three times with 5 mL of dry N,N-dimethylformamide (DMF). Then a mixed solution (1 mL of DMF) of the first Fmoc-protected amino acid (2eqv. Fmoc-D-Lys-Boc-OH) and N,N-diisopropylethylamine (DIPEA) was added, and the solution was bubbled through the solution. The bubble reacts the resin with the amino acid C-terminus for 40 minutes. The resin was then washed three times with DMF 5mL. The blocking solution (16:3:1 DCM / MeOH / DIPEA) was then added to ...
Embodiment 3
[0113] This example is used to illustrate the synthesis of 2-naphthaleneacetic acid-(D)-Phe-(D)-Phe-(D)-Lys-(benzenesulfonamide)-OH.
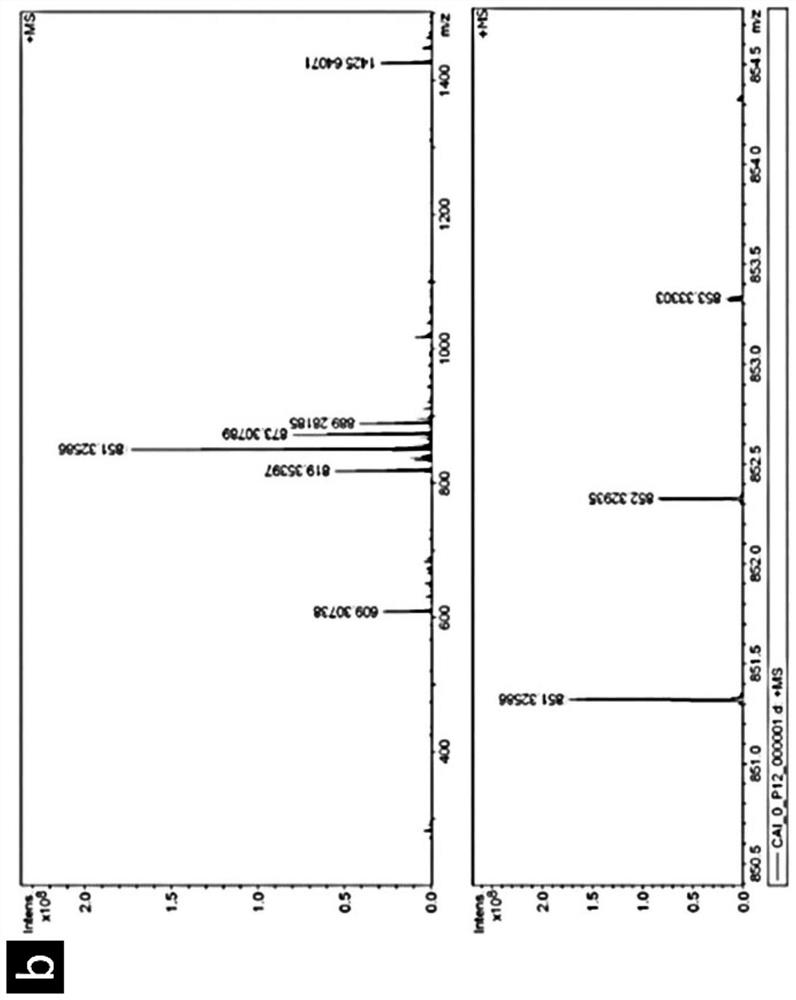
[0114] 0.3 mmol of the short peptide small molecule prepared in Example 2 above was added to methanol (6 mL) and dd H 2 0 (1 mL), then 1 M NaOH was added to adjust the pH to 8, the activated isothiocyanate-benzenesulfonamide (0.33 mmol) was dissolved in methanol (1 mL) and added to the short peptide small molecule in the mixed solution. The mixture was stirred at room temperature overnight. The obtained crude product was purified by HPLC.
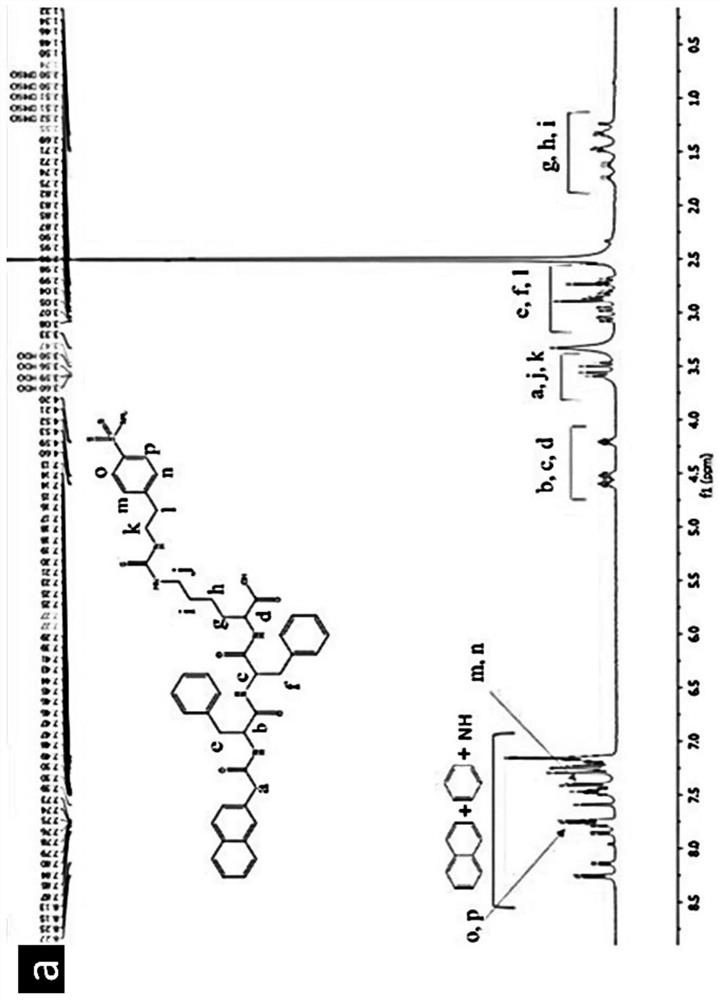
[0115] The NMR information is as follows: 1 H NMR (400MHz, DMSO-d 6 )δ8.25(d,J=8.1Hz,1H), 8.13(d,J=8.3Hz,1H),7.85(d,J=7.4Hz,1H),7.80–7.76(m,1H),7.75( d, J = 2.9Hz, 2H), 7.73 (d, J = 3.5Hz, 2H), 7.58 (s, 1H), 7.50–7.44 (m, 2H), 7.43 (d, 2H), 7.30–7.12 (m ,10H),4.61–4.55(m,2H),4.54–4.46(m,1H),4.20(dd,1H),),3.59(s,2H),3.53(dd,J=35.9,14.0Hz,2H ), 3.08–3.03 (m, 2H), 2.95–2.79 (m, 2H), 2.69 (dd, J=13.4, 10....
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com