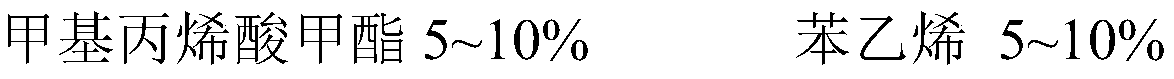Preparation method of acrylic resin for flexible plastics
A technology of acrylic resin and flexible plastic, which is applied in the field of resin to achieve the effect of improving flexibility, increasing reaction and good hydrolysis resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) First, put caprolactone and hydroxyethyl acrylate in an equimolar ratio into the reaction kettle, feed N2 protection to start stirring, and raise the temperature to 140-150°C to maintain this temperature;
[0022] (2) add an appropriate amount of organotin catalyst, maintain at this temperature for 5 hours, lower the temperature and cool down, and obtain intermediate B;
[0023] (3) 10% intermediate B, 8% (meth)methyl acrylate, 25% butyl methacrylate, 9% styrene, 9.5% hydroxyethyl acrylate, 0.8% acrylic acid, 3% initiator, Throw it into the high-level tank to dissolve safely and stir evenly;
[0024] (4) Add 35% xylene into the reaction kettle, raise the temperature to 130±2°C, drop the monomer in the head tank into the reaction kettle at a constant speed for 3 hours, keep it for 1 hour, add the initiator, and keep it for another 2 hours, and check the acid value of the resin , viscosity, and pass the test, dilute it with xylene to the required solid content, cool ...
Embodiment 2
[0026] (1) First, put caprolactone and hydroxyethyl acrylate in an equimolar ratio into the reaction kettle, feed N2 protection to start stirring, and raise the temperature to 140-150°C to maintain this temperature;
[0027] (2) add an appropriate amount of organotin catalyst, maintain at this temperature for 5 hours, lower the temperature and cool down, and obtain intermediate B;
[0028] (3) 15% intermediate B, 8.2% (meth)methyl acrylate, 22% butyl methacrylate, 10% styrene, 8% hydroxyethyl acrylate, 1.3% acrylic acid, 2.5% initiator, Throw it into the high-level tank to dissolve safely and stir evenly;
[0029] (4) Add 33% xylene into the reaction kettle, raise the temperature to 130±2°C, drop the monomer in the head tank into the reaction kettle at a constant speed for 3 hours, keep it for 1 hour, add the initiator, and keep it for another 2 hours, and check the acid value of the resin , viscosity, and pass the test, dilute it with xylene to the required solid content, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com