Carbonyl reductase mutant, recombinant expression carrier and application of carbonyl reductase mutant and recombinant expression carrier to production of chiral alcohol
An expression vector and reductase technology, applied in the field of enzyme engineering, can solve the problems of harsh reaction conditions, difficult operation, residual heavy metals, etc., and achieve the effects of easy preparation, environmental friendliness and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
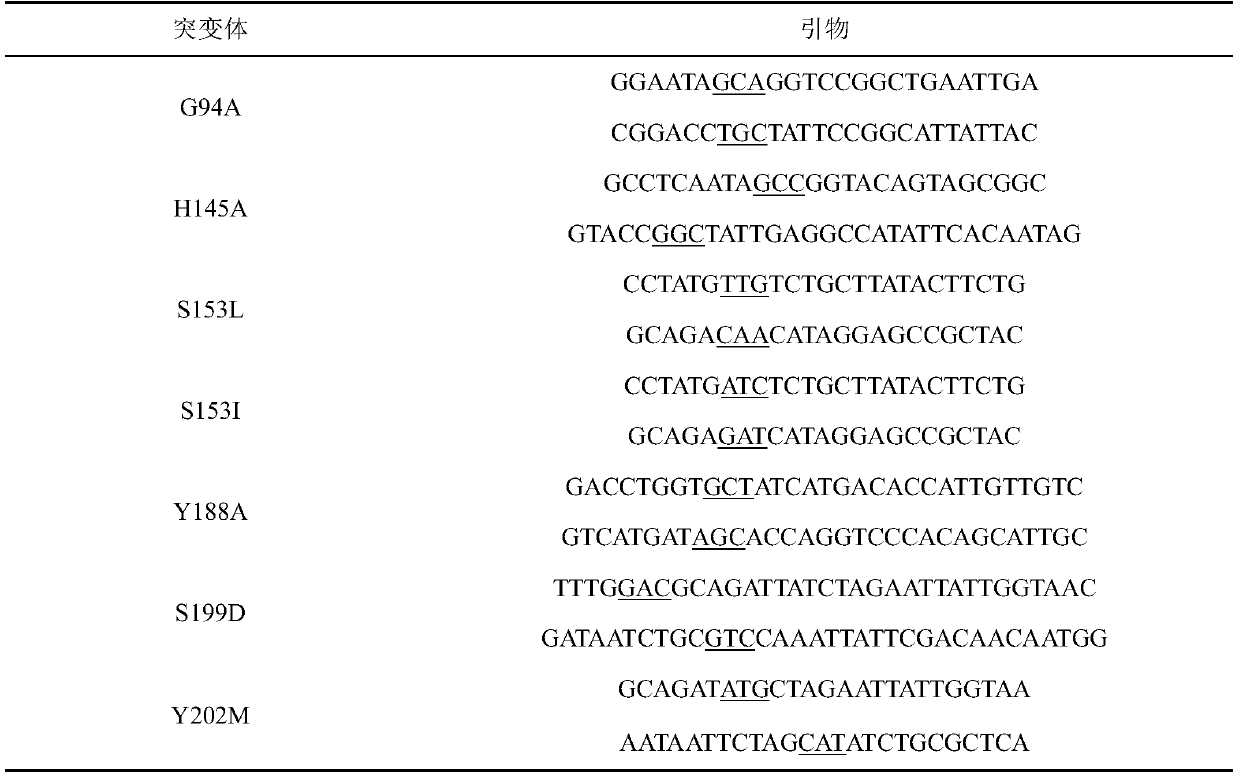
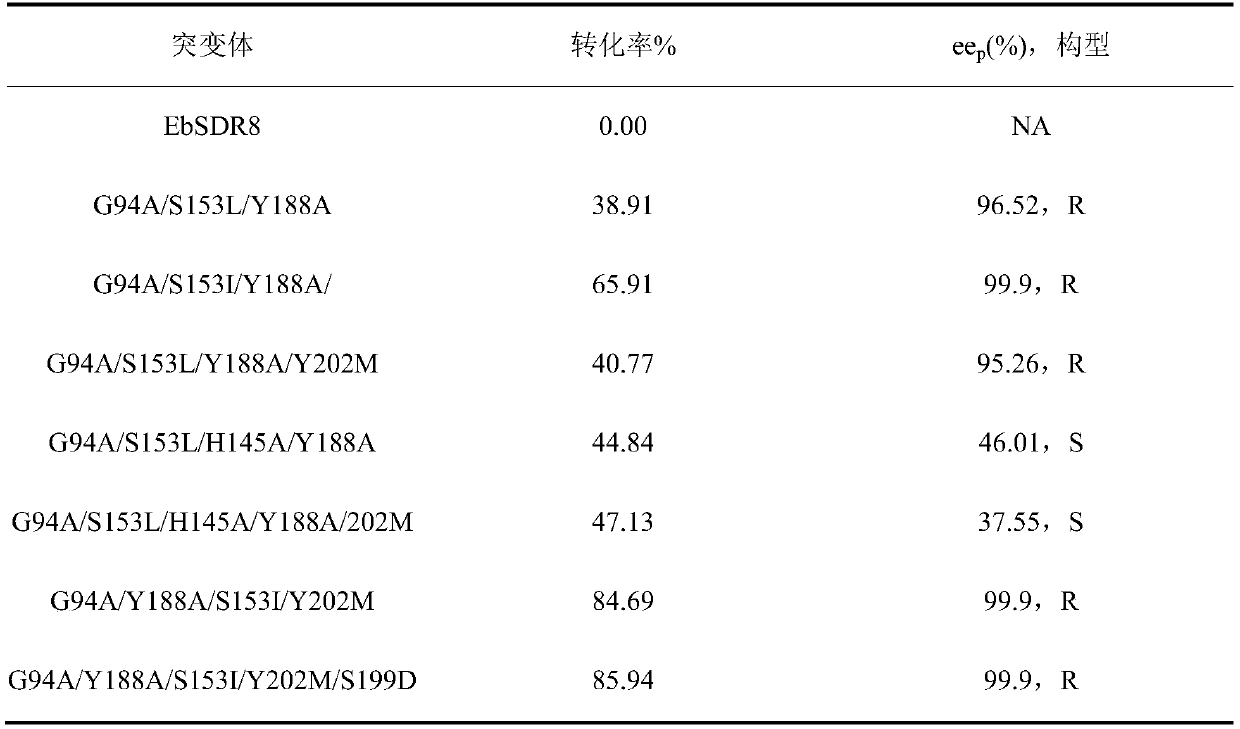
[0027] Example 1, replace the 94th glycine with alanine, the 153rd serine with leucine, and the 188th tyrosine in the amino acid sequence shown in the sequence listing as SEQ ID No.2 Alanine;
Embodiment 2
[0028] Example 2, replace the 94th glycine with alanine, the 188th tyrosine with alanine, and the 153rd serine in the amino acid sequence shown in the sequence listing as SEQ ID No.2 Valine;
Embodiment 3
[0029] Example 3, replacing the glycine at position 94 with alanine, the serine at position 153 with leucine, and the tyrosine at position 188 with Alanine, tyrosine at position 202 is replaced by methionine;
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com