cystestermerol A and its application in improving diabetes
A diabetes drug and hypoglycemic technology, applied in the field of medicine, can solve the problem of very few active ingredients research, and achieve the effects of improving insulin resistance, inhibiting activity, and promoting glucose consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The extraction and identification of embodiment 1 Cysestermerol A
[0042] 1. Extraction method
[0043] Bermudagrass is extracted with 95% ethanol aqueous solution to obtain extract extract, which is sequentially extracted with petroleum ether, ethyl acetate and n-butanol to obtain ethyl acetate extract; the ethyl acetate extract is loaded on a silica gel chromatographic column (200~ 300 mesh), different proportions of CH 2 Cl 2 / Acetone gradient elution gives 5 fractions Fr.1, Fr.2, Fr.3, Fr.4, Fr.5; after testing the target compound is concentrated in Fr.5 (CH 2 Cl 2 : acetone=1:1), Fr.5 was subjected to MCI gel CHP 20P column chromatography, SephadexLH-20 gel column chromatography and semi-preparative chromatography to obtain the target monomer compound, denoted as Cysestermerol A.
[0044] 2. Structural identification method
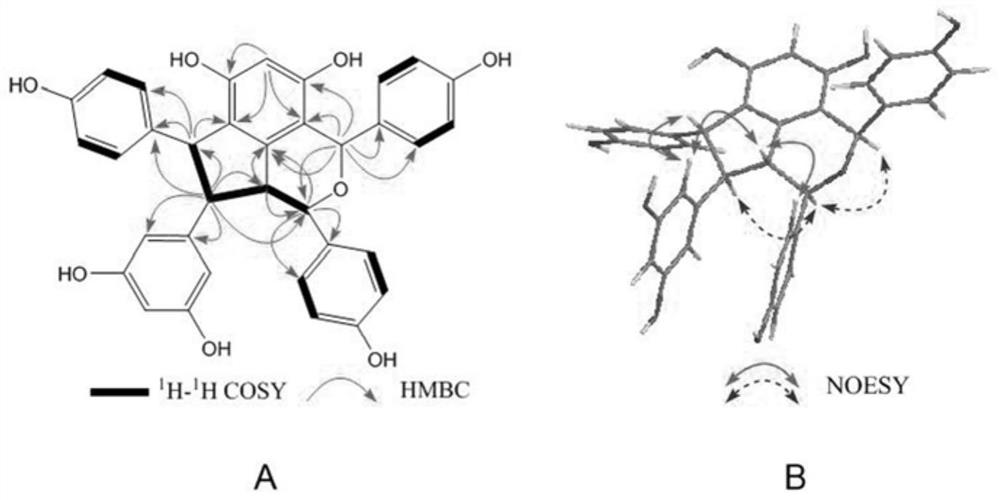
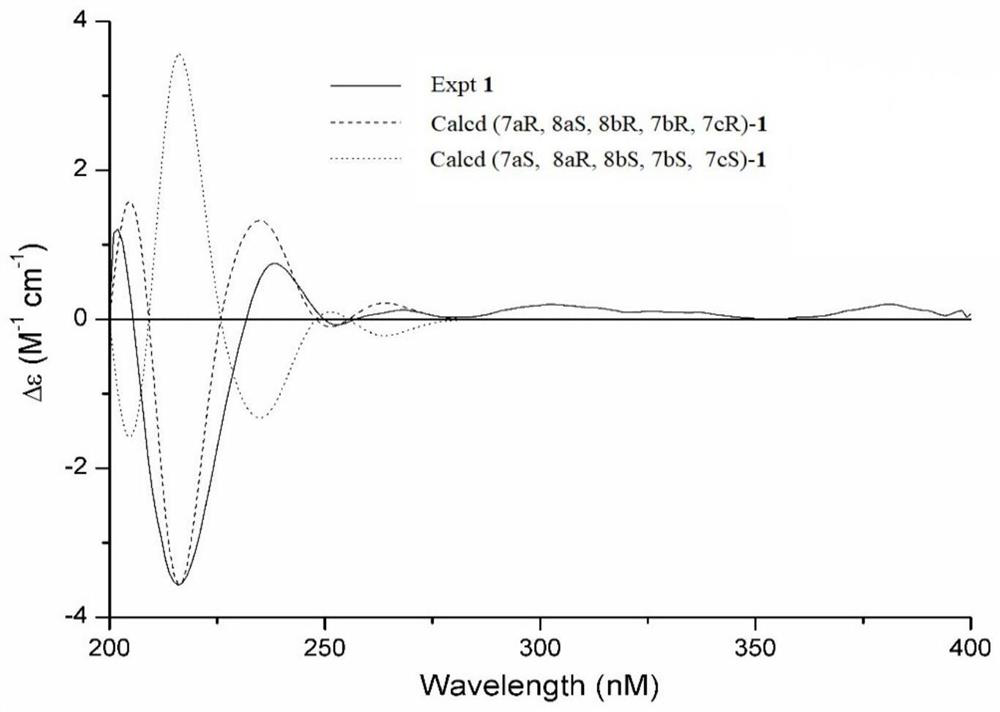
[0045] The planar structure and relative configuration of Cysestermerol A were determined by HR-ESI-MS and NMR, and its absolute config...
Embodiment 2
[0056] Example 2 The improvement effect of Cysestermerol A on IR-HepG2 cells
[0057] 1. HepG2 cell culture
[0058] After recovery, HepG2 cells were cultured in DMEM high-glucose medium containing 10% fetal bovine serum in a 25cm 2 culture flasks, stored in constant temperature CO 2 37°C in an incubator, 5% CO 2 cultivated under conditions. When the cells adhered to 90%, they were washed twice with PBS, digested with 0.25% trypsin, passaged at a ratio of 1:3, and the cells in the logarithmic growth phase were used for the experiment.
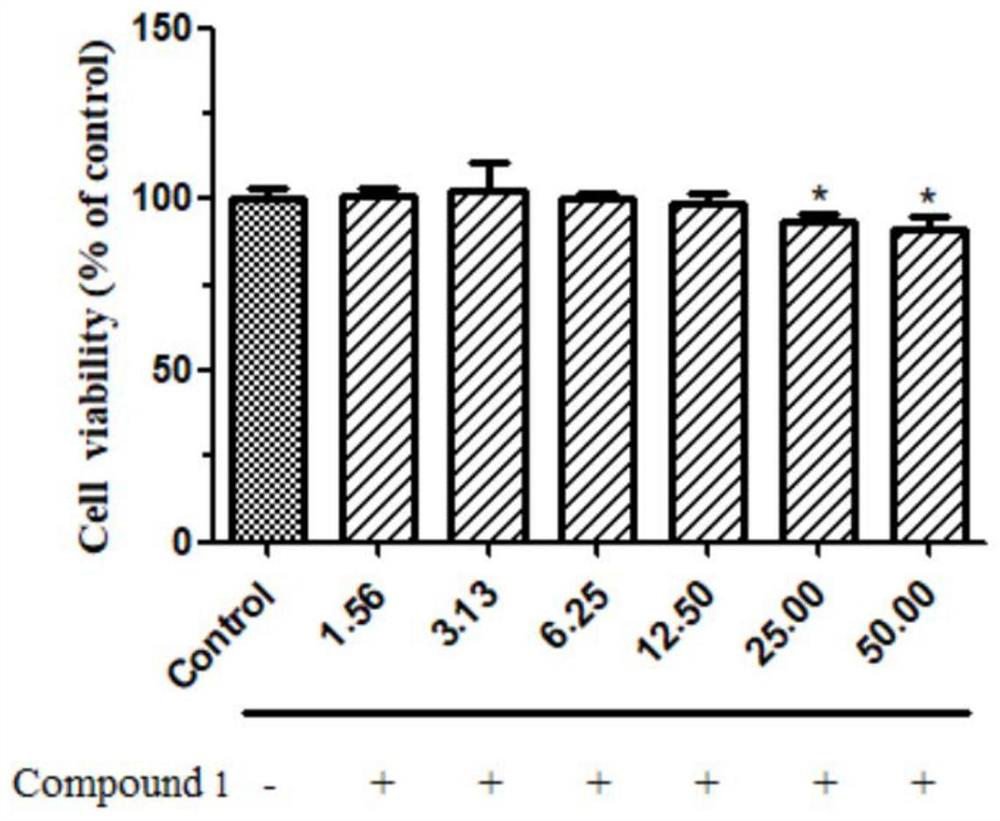
[0059] 2. Determination of the safety range of drug administration (CCK8 method)
[0060] (1) Experimental method
[0061] HepG2 cells in the logarithmic growth phase were mixed with 3x10 4 Cells were seeded in a 96-well plate at 37°C, 5% CO 2 nourish. After the cells adhered to the wall, Cysestermerol A with a concentration gradient of 50, 25, 12.5, 6.25, 3.125, and 1.5625 μmol / L diluted with DMEM culture solution containing 10% FBS wa...
Embodiment 3
[0074] The influence of embodiment 3 Cysestermerol A on α-glucosidase inhibition
[0075] 1. Experimental method
[0076] Weigh (Na 2 HPO 4 )12H 2 0 3.58g dilute to 1000mL with distilled water as liquid A; weigh KH 2 PO 4 1.36g was made up to 1000mL with distilled water as solution B; take 49.6mL of solution A and 50.4mL of solution B, mix evenly, set the volume to 1000mL, pipette 100mL from it and then set the volume to 1000mL, adjust the pH to 6.8 to get 0.1mmol / L PB solution.
[0077] Both the substrate PNPG and α-glucosidase were dissolved in PB solution at concentrations of 20 mmol / L and 0.1 mg / ml. Take 0.5mL α-glucosidase solution and a certain amount of sample solution to be tested, supplement the buffer solution until the volume of the mixed solution is 3.5mL, mix well, incubate in a constant temperature water bath at 37°C for 10min, add 0.5mL PNPG, mix well to start the reaction, and 37°C constant temperature reaction for 30min, then add 1mol / L Na 2 CO 3 The...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com