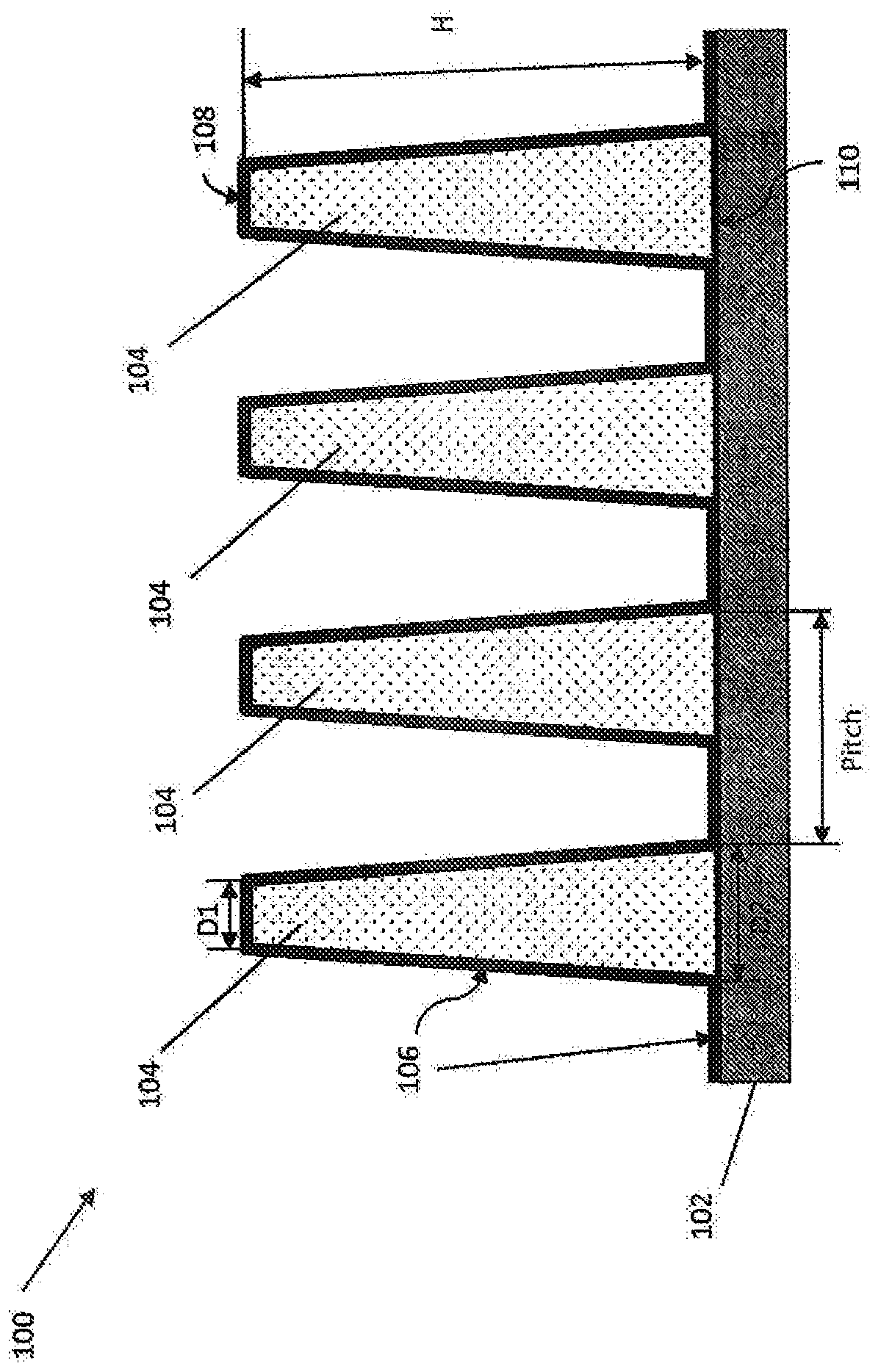
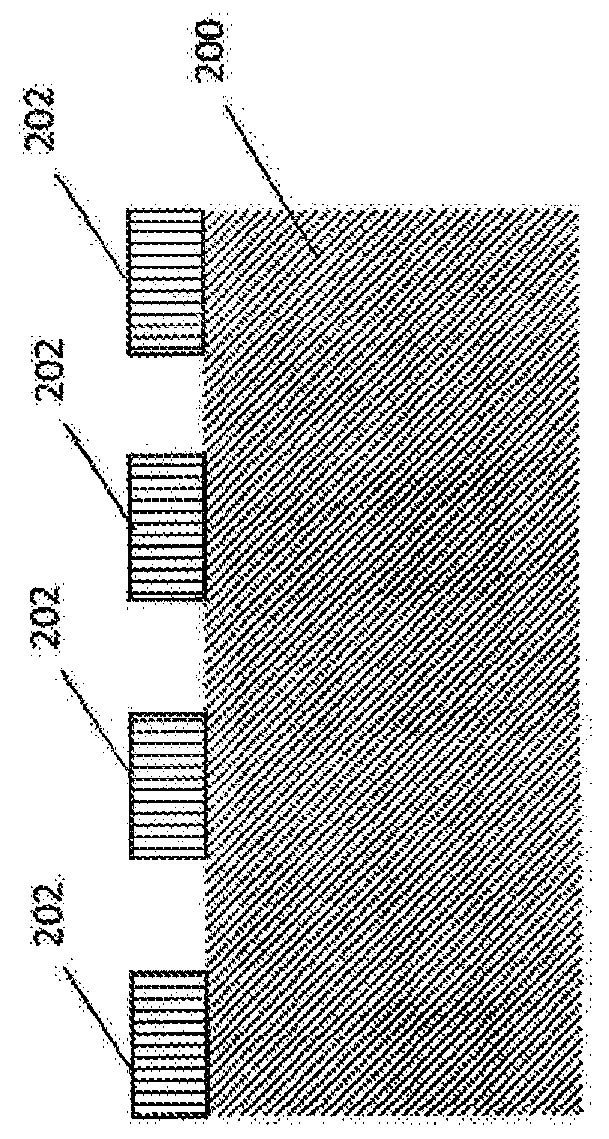
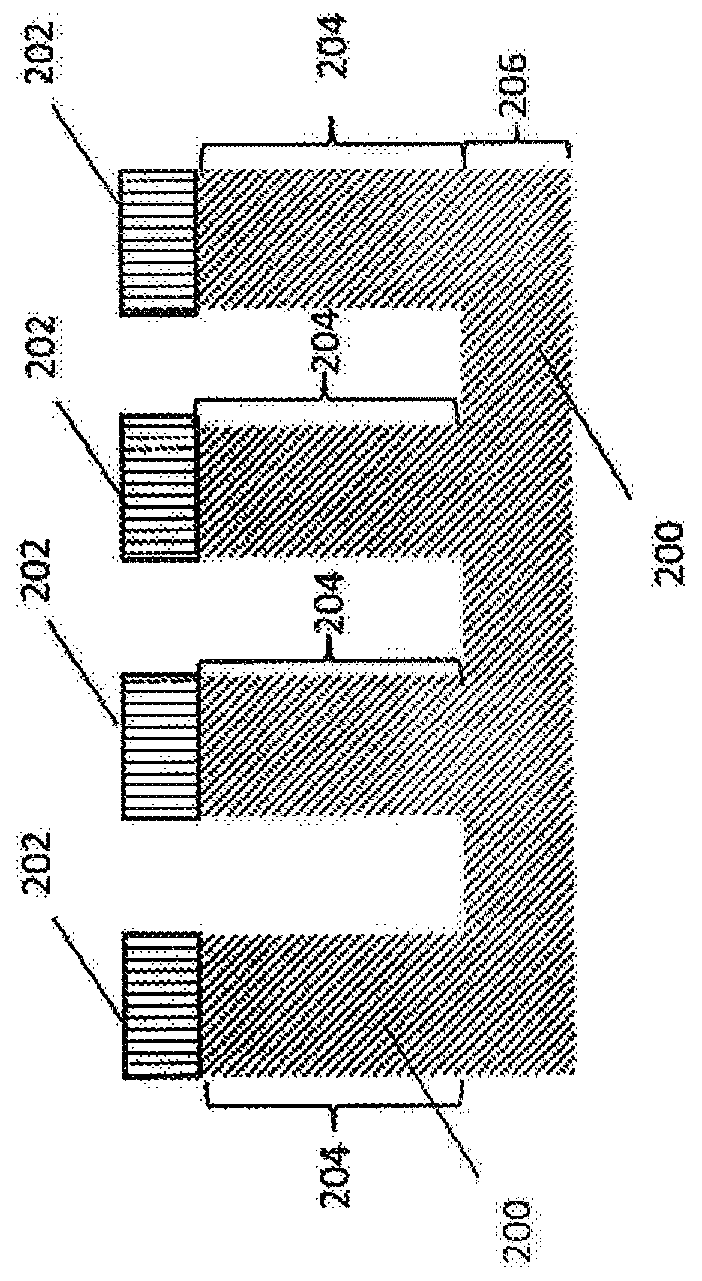
Antibacterial medical implant surface
A technology for implanting devices and photoresist layers, which can be used in drug delivery, pharmaceutical formulations, medical science, etc., and can solve problems such as random structural patterns, sizes and specifications that are not suitable for medical implants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] Medical implants are becoming more common as new technologies become available to treat ailments associated with disease and aging. However, complete sterilization of implanted devices by conventional means is difficult, leading to the introduction of infectious substances into the body along with surfaces that can provide a bacterial matrix to form biofilms. Not only can this biofilm lead to potentially serious infection and disease, but it often requires surgery to remove the infectious material from the body. In addition, the growth of bacterial films on the surface of implanted medical devices, including, for example, pacemakers and orthopedic implants, can also prevent the proper operation of the device and sometimes lead to device failure.
[0033] There is a need to reduce or eliminate bacterial infections associated with medical devices to improve patient outcomes. Special considerations, such as biocompatibility, may be required to prevent infection. Conventi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Average height | aaaaa | aaaaa |
| Average spacing | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com