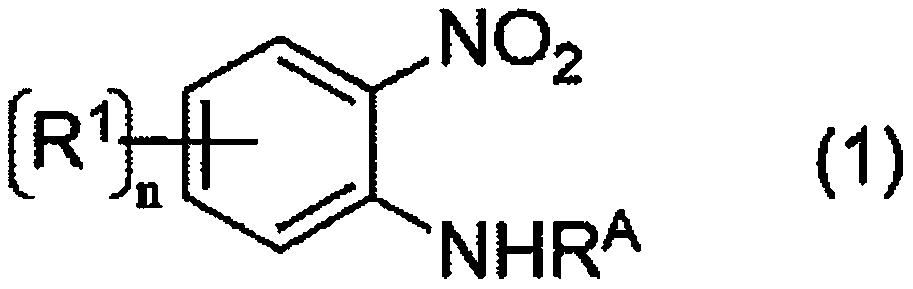
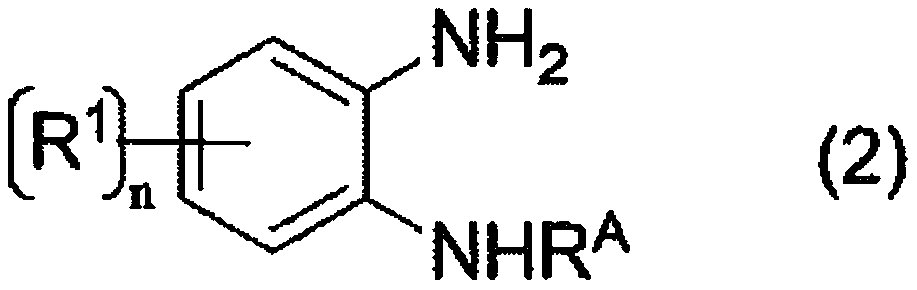
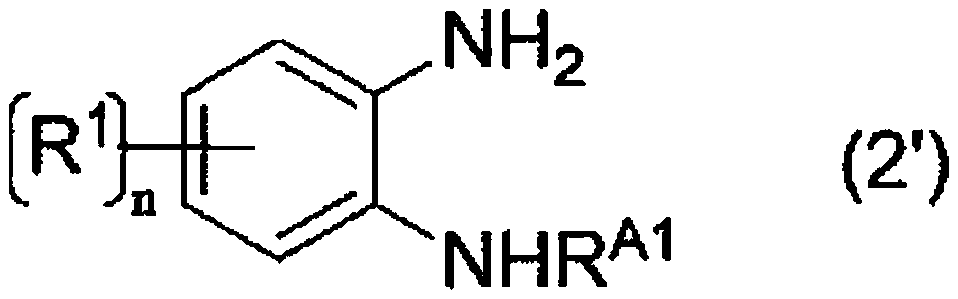
Method for producing diaminobenzene compound
A kind of technology of diaminobenzene and compound is applied in the field of diaminobenzene compound to achieve the effects of improving yield, promoting dissolution and increasing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0183] Example 1 (Example of producing diaminobenzene compound using aminonitrobenzene compound)
[0184] The reaction of the following formula is carried out.
[0185]
[0186] Methyl 2-amino-3-nitrobenzoate (1.0 g, 5.1mmol; 2-amino-3-nitrobenzene compound), sodium dithionite (purity 80% by mass) (3.33g, 15.3mmol ((the molar number of sodium dithionite calculated from the purity); Wako Pure Chemicals Produced by the company, alkali metal dithionite), potassium carbonate (1.4g, 10.2mmol; alkali metal carbonate) were stirred and mixed at a reaction temperature of 100°C for 4 hours (reaction time).
[0187]Water (14 mL) was added to the obtained reaction liquid, and then, a mixed liquid of the obtained reaction liquid and water was extracted with ethyl acetate (5 mL). The extraction based on this ethyl acetate (5 ml) was repeated 5 times. After washing this ethyl acetate solution (total 25ml), by concentrating under reduced pressure, obtain target product 2,3-diaminobenzoi...
Embodiment 2
[0190] Example 2 (Example of producing diaminobenzene compound using aminonitrobenzene compound)
[0191] With respect to embodiment 1, except using sodium dithionite (purity 80% by mass) (2.22g, 10.2mmol ((the molar number of sodium dithionite calculated from purity); Wako Pure Chemical Company produces, the alkali of dithionite Metal salt), except that the reaction time was 5 hours, others carried out the same operation as Example 1. The conversion rate was 10%. Polar impurities were not confirmed.
Embodiment 3
[0192] Example 3 (Example of producing diaminobenzene compound using aminonitrobenzene compound)
[0193] With respect to Example 1, except that the reaction solvent was a mixed solvent of DMF (7ml) and water (0.099ml, 5.1mmol), the same operation was performed as in Example 1. The yield of methyl 2,3-diaminobenzoate was 94%. The conversion rate was 100%. In addition, the purity of methyl 2,3-diaminobenzoate confirmed by HPLC was 95.4%, and the polar impurities were 2.5%. The analytical results of the obtained methyl 2,3-diaminobenzoate were the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com