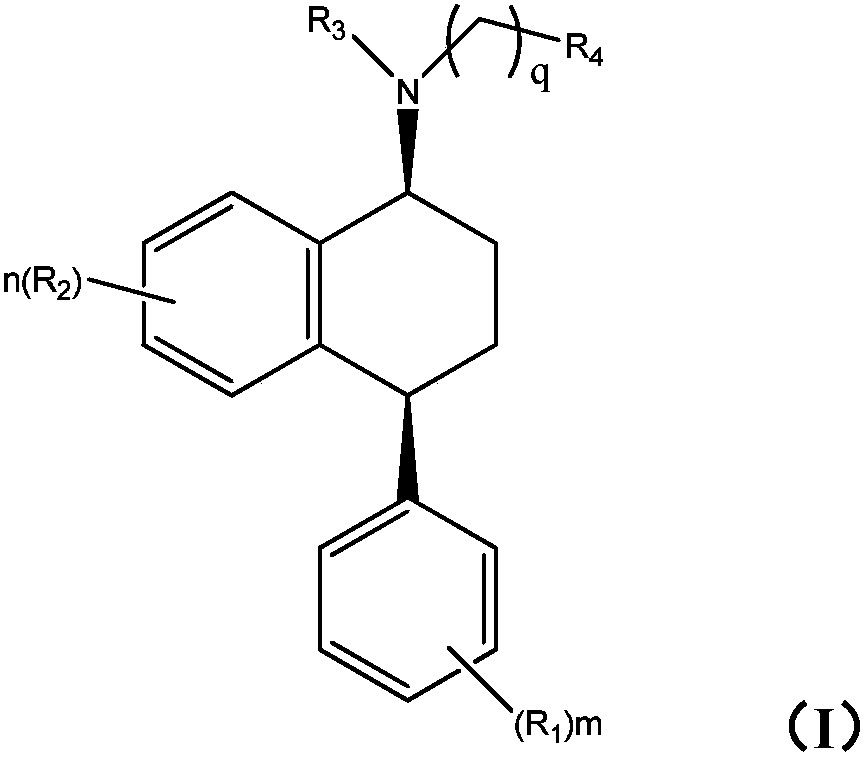
New sertraline analogue, preparation method and applications thereof
A kind of analog, technology of sertraline, applied in the field of compound and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
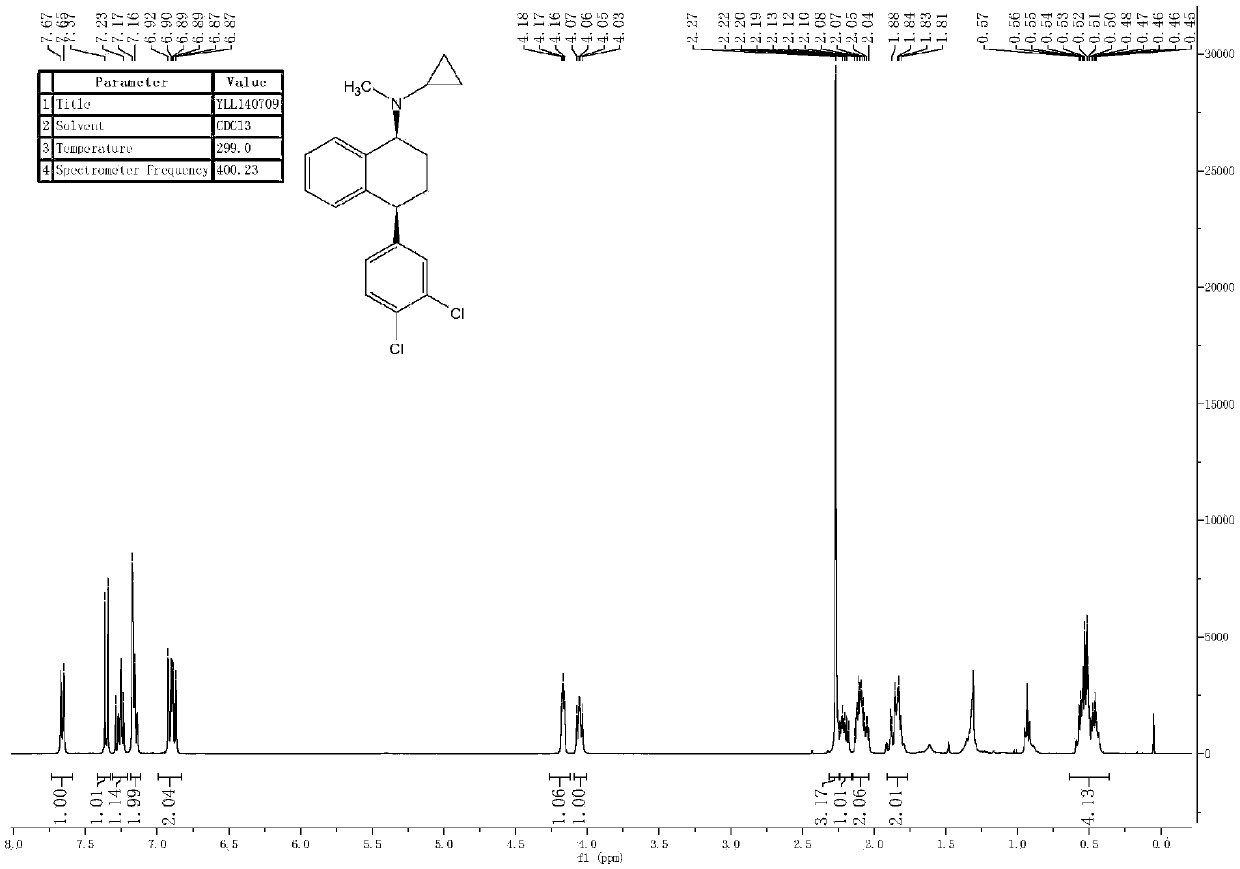
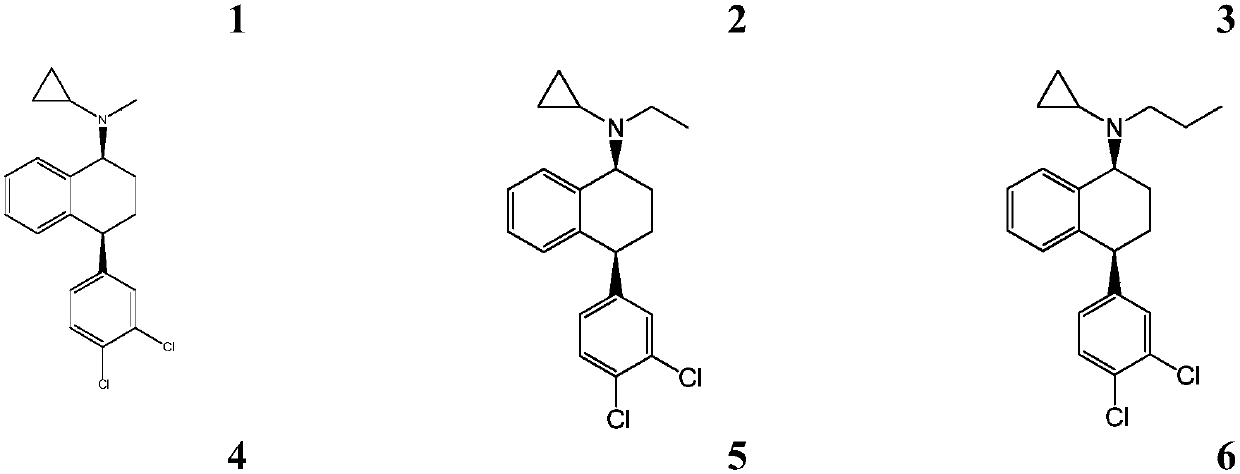
[0096] Embodiment 1: preparation compound 1
[0097]
[0098] Add the compound of formula 1-S (12g, 0.035mol, 1eq) into a 250ml single-necked flask, and 150ml of water as a solvent. Under stirring, add excess potassium carbonate to it, and stir at room temperature for 1-2h.
[0099] Post-processing: extract with 200ml*2EA, dry over anhydrous magnesium sulfate, concentrate by suction filtration, and obtain 10.9g of pure product, which is directly used in the next step without post-processing.
[0100]
[0101] Add the above-mentioned pure product (10.9g, 0.0356mol, 1eq) into a 250ml two-necked flask, 100ml of a mixed solvent of methanol and tetrahydrofuran (volume ratio 1:1) as a solvent, and add 1-ethoxy-1 Trimethylsilylcyclopropane (7.5g, 0.0427mol, 1.2eq) and acetic acid (4.3g, 00712mol, 2eq), and finally, sodium cyanoborohydride (4.5g, 0.0712mol, 2eq) was added in portions, and the addition was complete Then, heat to reflux overnight.
[0102] Cool to room temperatu...
Embodiment 2
[0107] Embodiment 2: drug activity test
[0108] 2.1 Determination of antidepressant activity by mouse tail suspension test
[0109] Male ICR mice (SPF grade), weighing 18-22g, attach a clip to the center rope of the top plate of a 25×25×25cm tail-suspension box, clamp the mouse’s tail tip 1cm to make it hang upside down, and the head is 4-5cm away from the bottom of the box . A certain dose (24 mg / kg) of the exemplary compound of the present invention, sertraline, and 0.5% sodium carboxymethylcellulose solution was ground to prepare a suspension for use. Different doses of samples to be tested were orally administered 60 minutes before the experiment, and the tail was suspended for 6 minutes after administration, and the immobility time of the mice was accumulated for 4 minutes after the experiment. The results are shown in Table 2.
[0110] 2.2 Determination of antidepressant activity by forced swimming test in mice
[0111] Male ICR mice (SPF grade), weighing 18-22 g, w...
Embodiment 3
[0114] Acute Toxicity Study of Exemplary Example Compounds of Example 3
[0115] Experimental Materials:
[0116] ICR mice, half male and half female, weighing 18-22 g, were fed with pellet feed, and had free access to food and water.
[0117] The compounds tested in Table 2 were respectively prepared into suspensions with a concentration of 500 mg / mL with 0.5% CMC-Na.
[0118] experimental method
[0119] The ICR mice were given the compounds tested in Table 2 at a dose of 10 ml / kg by body weight once, and the toxicity and death of the animals within 14 days after administration were observed. It was found that after a single intragastric administration of the mice, the mice moved sluggishly, lay still and moved less, and returned to normal after 40-60 minutes. Within 14 days after the administration, the mice did not die. On the 15th day, all the mice were sacrificed, dissected, and all organs were inspected with the naked eye, and no obvious lesions were found.
[0120]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum tolerated dose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com