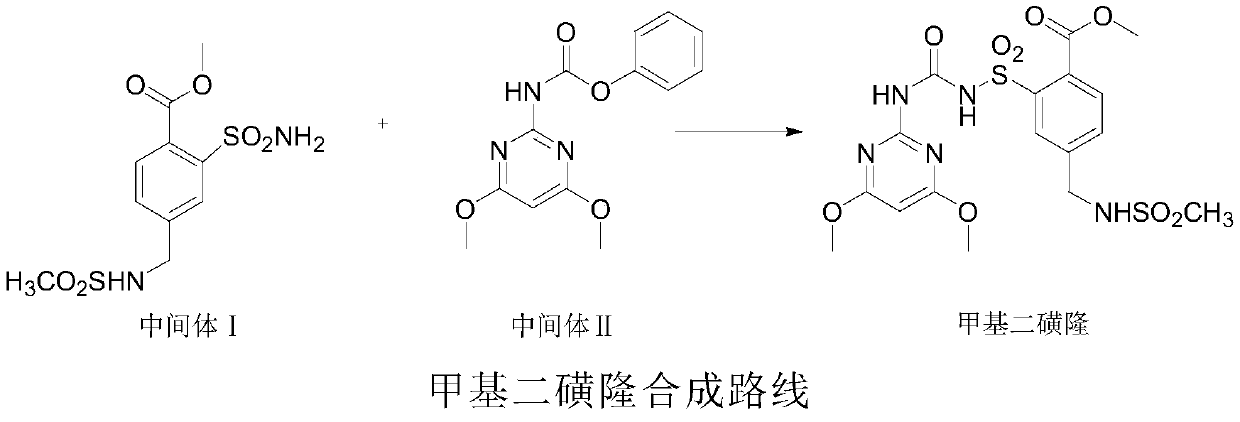
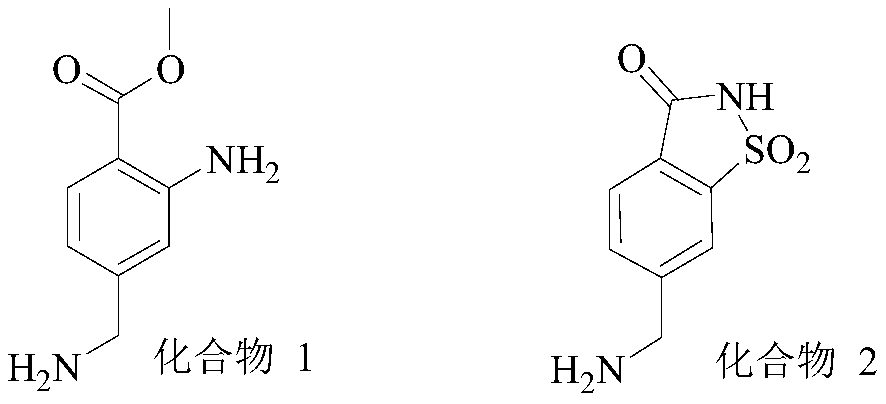
2-(4-methylbenzyl)-1H-isoindole-1,3-dione derivative and synthesis method thereof
A technology of methyl benzyl and synthetic method, which is applied in the field of organic drug synthesis, can solve the problems of troublesome synthetic method and low yield, and achieve the effects of reduced production cost, high yield and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This embodiment provides the synthesis method of intermediate 4, intermediate 5, intermediate 6 and corresponding intermediates and the method of synthesizing compound 2 by using intermediate 4, intermediate 5 and intermediate 6.
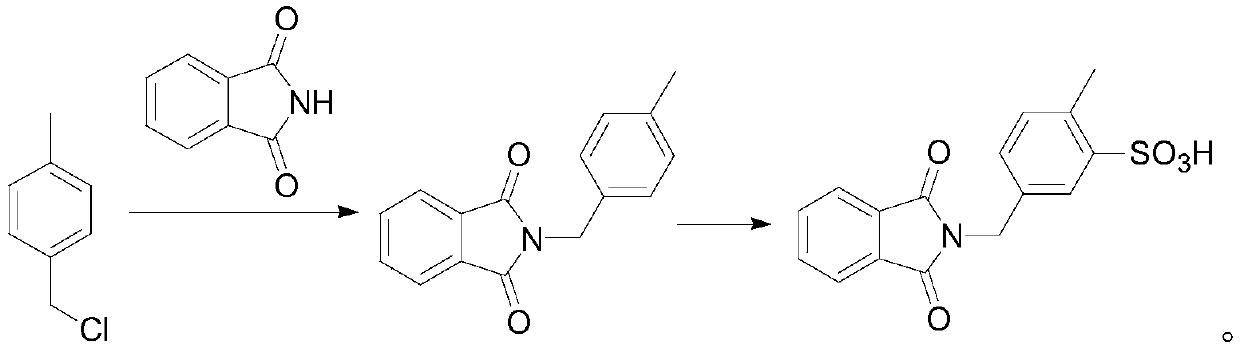
[0042] Intermediate 4: 2-(3-Sulphono-4-methylbenzyl)-1H-isoindole-1,3-dione
[0043] The synthetic method of described intermediate 4 comprises the following steps:
[0044] Synthesis of 2-(4-methylbenzyl)-1H-isoindole-1,3-dione Weigh 7g of p-methylbenzyl chloride and dissolve it with an appropriate amount of N,N-dimethylformamide, and add it to the system successively Add 7.35g of phthalimide, 8.1g of tetrabutylammonium bromide and 5.3g of sodium carbonate, and react at 50°C for 2h; after the reaction, extract with water, collect the organic layer and remove the solvent to obtain 12.3g of 2- (4-methylbenzyl)-1H-isoindole-1,3-dione;
[0045] Synthesis of Intermediate 4 Dissolve 12.3g of 2-(4-methylbenzyl)-1H-isoindole-1,3-dione in chlorofo...
Embodiment 2
[0065]This embodiment provides the synthesis method of intermediate 4, intermediate 5, intermediate 6 and corresponding intermediates and the method of synthesizing compound 2 by using intermediate 4, intermediate 5 and intermediate 6.
[0066] Intermediate 4: 2-(3-Sulphono-4-methylbenzyl)-1H-isoindole-1,3-dione
[0067] The synthesis method of intermediate 4 in this example is basically the same as the synthesis method of intermediate 4 provided in Example 1, the main difference is that the reaction solvent, basic catalyst and reaction parameters are different. Specifically, in this example, 7g of p-methylbenzyl chloride was weighed and dissolved with an appropriate amount of pyridine, and 7.35g of phthalimide, 16g of tetrabutylammonium bromide and 6g of sodium hydroxide were sequentially added to the system, React at 100°C for 1 hour; after the reaction, extract with water, collect the organic layer and remove the solvent to obtain 12.1 g of 2-(4-methylbenzyl)-1H-isoindole-...
Embodiment 3
[0079] This embodiment provides intermediate 4, intermediate 6 and the corresponding intermediate synthesis method and the method of using intermediate 4 and intermediate 6 to synthesize compound 2.
[0080] Intermediate 4: 2-(3-Sulphono-4-methylbenzyl)-1H-isoindole-1,3-dione
[0081] The synthesis method of intermediate 4 in this example is basically the same as the synthesis method of intermediate 4 provided in Example 1, the main difference is that the reaction solvent, basic catalyst and reaction parameters are different. Specifically, in this example, 7g of p-methylbenzyl chloride was weighed and dissolved with an appropriate amount of dimethyl sulfoxide, and 7.35g of phthalimide, 3.5g of tetrabutylammonium bromide and 6g of triethylamine was reacted at 70°C for 1.5h; after the reaction was completed, it was extracted with water, and the organic layer was collected to remove the solvent to obtain 12.2g of 2-(4-methylbenzyl)-1H-isoindole-1,3- Diketone;
[0082] Dissolve...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com