Antitussive solution preparation for inhalation and preparation method of solution preparation
A preparation and solution technology, applied in the field of pharmacy, can solve the problems of difficult swallowing, poor patient compliance, long disintegration time, etc., and achieve the effects of avoiding the first-pass effect, high drug concentration, and no toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
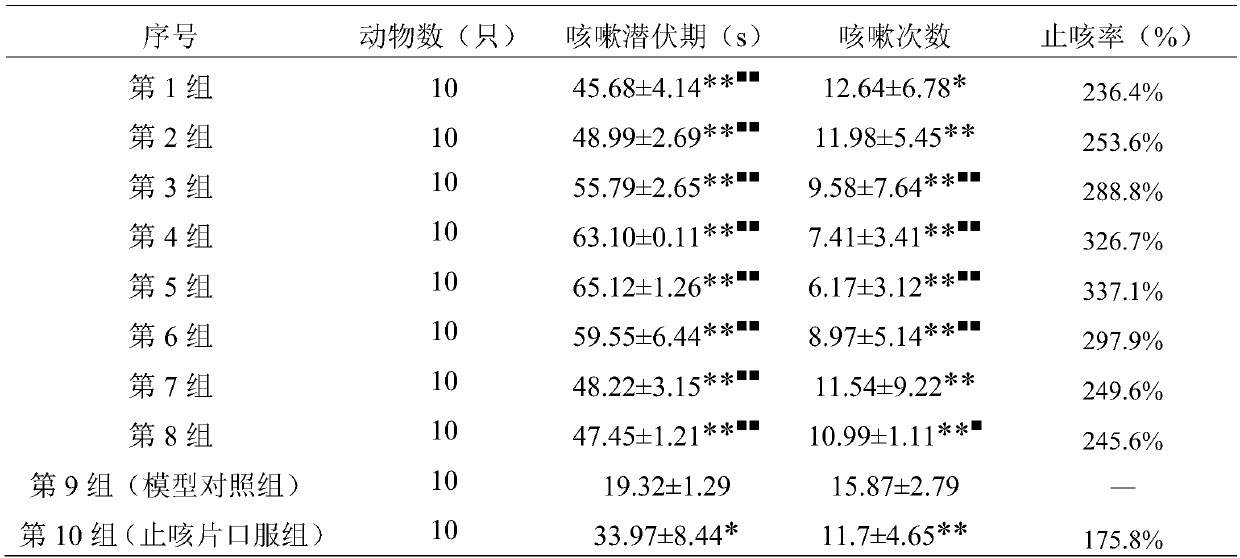
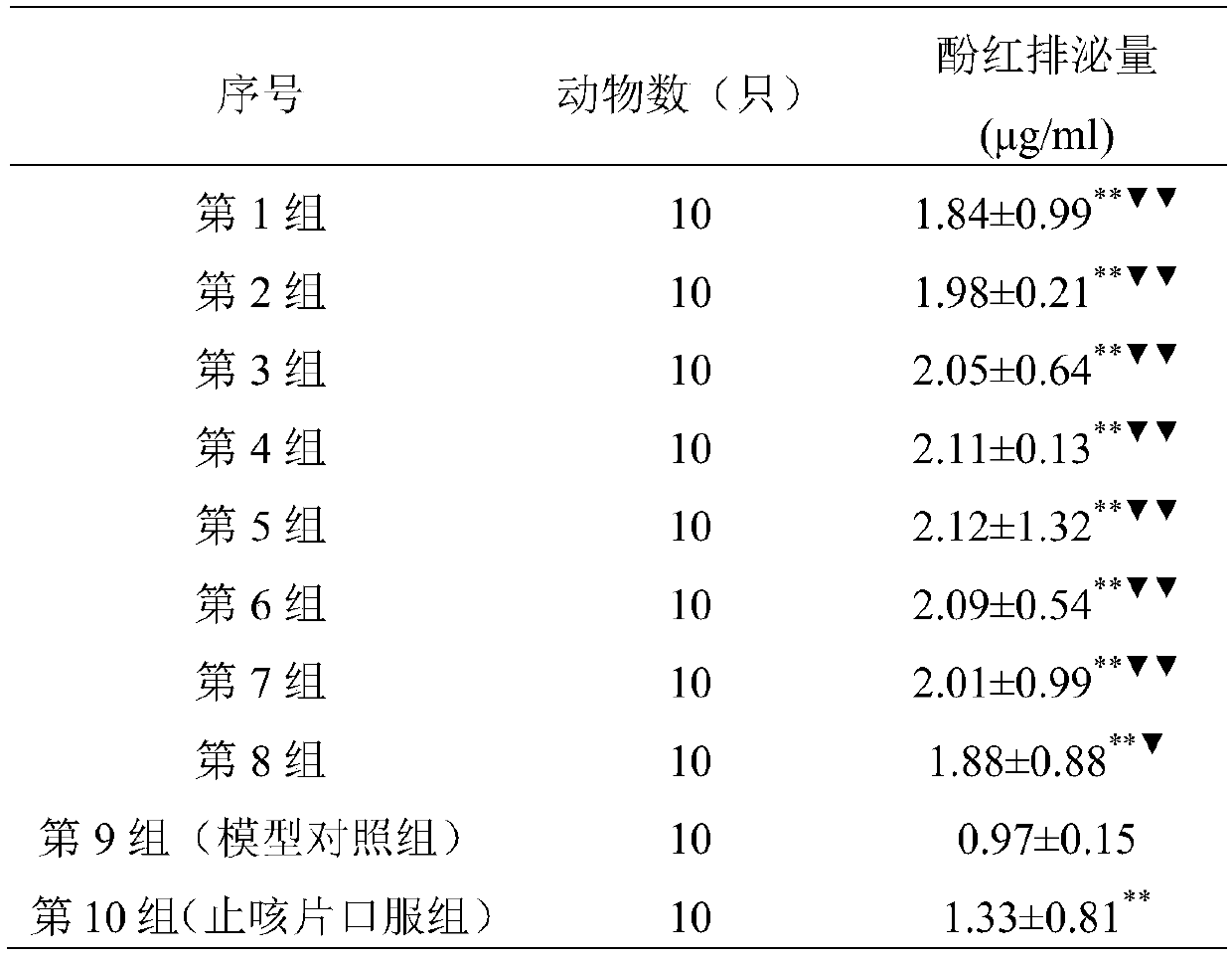
[0041] Cough solution for inhalation 1000ml:
[0042] Baibu 100g
[0043] Peucedanum 106g
[0044] Bitter almonds 80g
[0045] (1) Weigh the raw materials Baibu, Peucedanum, and Bitter Almond according to the prescription ratio;
[0046] (2) Take a hundred parts, add 8 times the amount of water for the first time, decoct for 1.5 hours, add 8 times the amount of water for the second time, decoct for 1.5 hours, filter, combine the filtrates, concentrate to 1ml containing 5g of crude drug, add ethanol to contain Alcohol content 70%, stir well, stand still for 24h, recover ethanol after filtration until 1ml contains 4g crude drug, add water until 1ml contains 1g crude drug, let stand for 24h, filter; concentrate until 1ml contains 5g crude drug, add ethanol to alcohol content 85% %, stir well, let stand for 12 hours, recover ethanol after filtration until 1ml contains 10g crude drug, add water until 1ml contains 1g crude drug, pass through macroporous resin column, wash with wa...
Embodiment 2
[0051] Cough solution for inhalation 1000ml:
[0052] 100 120g
[0053] Pedicure 135g
[0054] Bitter almonds 90g
[0055] (1) Weigh the raw materials Baibu, Peucedanum, and Bitter Almond according to the prescription ratio;
[0056] (2) Take a hundred parts, add 8 times the amount of water for the first time, decoct for 2 hours, add 8 times the amount of water for the second time, decoct for 1 hour, filter, combine the filtrate, concentrate to 1ml containing 4g crude drug, add ethanol to the alcohol content 70%, stir well, stand still for 12 hours, recover ethanol after filtration until 1ml contains 4g crude drug, add water until 1ml contains 3g crude drug, let stand for 24h, filter; concentrate until 1ml contains 5g crude drug, add ethanol until the alcohol content is 85%, Stir well, let stand for 12 hours, recover ethanol after filtration until 1ml contains 8g crude drug, add water until 1ml contains 0.5g crude drug, pass through macroporous resin column, wash with water...
Embodiment 3
[0061] Cough solution for inhalation 1000ml:
[0062] 100 165g
[0063] Pedicure 185g
[0064] Bitter almonds 105g
[0065] (1) Weigh the raw materials Baibu, Peucedanum, and Bitter Almond according to the prescription ratio;
[0066] (2) Take a hundred parts, add 8 times the amount of water for the first time, decoct for 2 hours, add 10 times the amount of water for the second time, decoct for 1.5 hours, filter, combine the filtrate, concentrate to 1ml containing 3g of crude drug, add ethanol to contain alcohol Amount of 70%, stir well, stand still for 12h, recover ethanol after filtration until 1ml contains 3.5g crude drug, add water until 1ml contains 0.6g crude drug, let stand for 24h, filter; concentrate until 1ml contains 4g crude drug, add ethanol to alcohol content 90%, stir well, let stand for 12h, recover ethanol after filtration until 1ml contains 11g crude drug, add water until 1ml contains 1.5g crude drug, pass through macroporous resin column, wash with water,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com