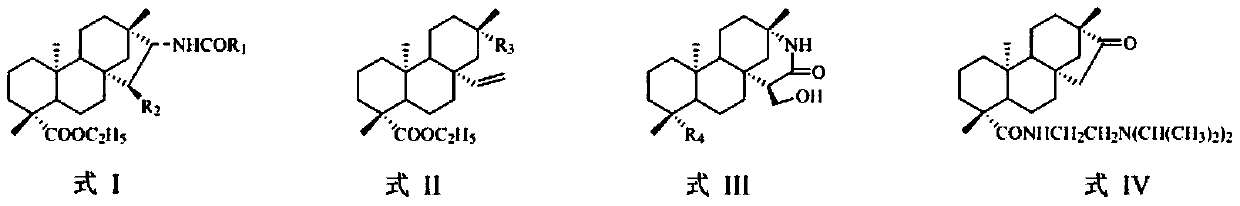
Isosteviol amide derivatives and preparation method therefor
A technology for isosteviol amide and its derivatives, which is applied in the field of isosteviol amide derivatives and its preparation, can solve problems such as rare reports, and achieve simple preparation methods, good application prospects, and high yields Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
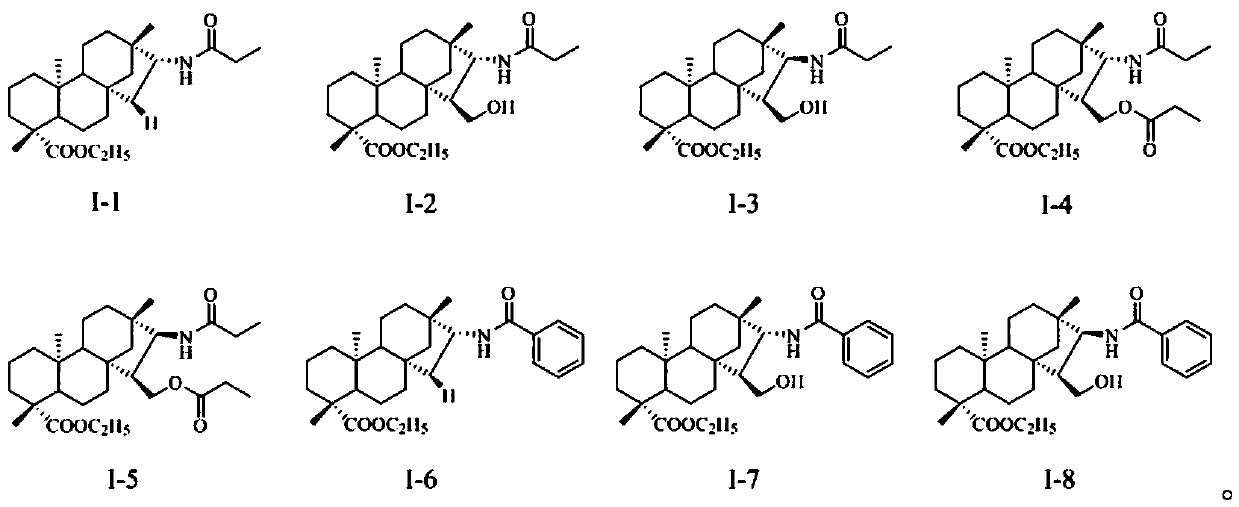
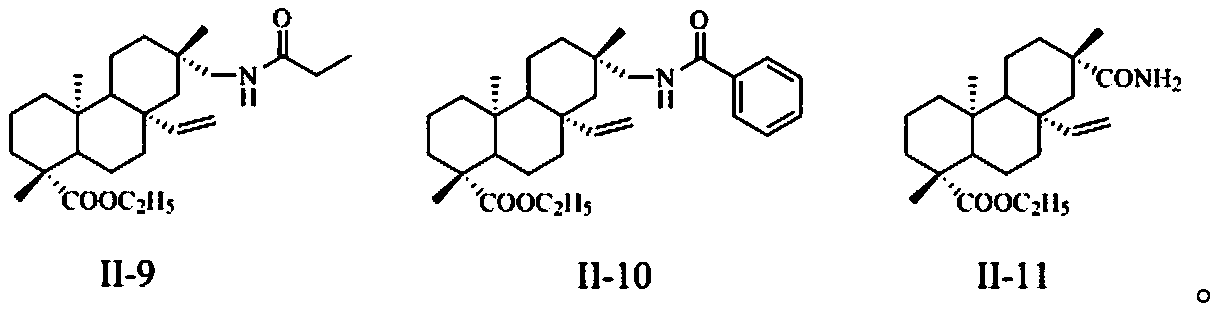
[0038] General method for the preparation of embodiment 1 compound I-1~I-3, II-9
[0039] Compounds 4, 8, 12, 14 (10 mmol) and propionic anhydride (11 mmol) were weighed and dissolved in 100 mL of pyridine solution, and the reaction was stirred at room temperature until the reaction was detected by TLC. A large amount of distilled water was added to the system, a white solid was precipitated, filtered, washed with water until neutral to obtain the crude compound, and recrystallized from ethanol to obtain the corresponding white solid compounds I-1~I-3 and II-9.
[0040]Among them, compounds 4, 8, 12, and 14 (refer to the paper European Journal of Medicinal Chemistry 115 (2016) 26-40 for synthetic conditions).
[0041]
[0042] Compound I-1: Yield: 89%; 1 H NMR (400MHz, CDCl 3, ppm): δ5.51(d, J=8.6Hz, 1H), 4.12–4.00(m, 2H), 2.22(q, J=7.6Hz, 2H), 2.16(d, J=13.3Hz, 1H), 1.91–1.77(m,3H),1.70–1.54(m,6H),1.40–1.29(m,5H),1.24(t,J=7.1Hz,3H), 1.16(t,J=7.6Hz,3H) ,1.15(s,3H),1.10–...
Embodiment 2
[0046] General method for the preparation of embodiment 2 compound I-4 and I-5
[0047] Compounds 8 and 12 (10 mmol) and propionic anhydride (22 mmol) were weighed and dissolved in 100 mL of pyridine solution, stirred at room temperature for reaction, and detected by TLC until the reaction was complete. A large amount of distilled water was added to the system, a white solid was precipitated, filtered, and the solid was washed with water until neutral to obtain the crude compound, which was recrystallized from ethanol to obtain the corresponding white solid compounds I-4 and I-5.
[0048] Compound I-4: Yield: 97%; 1 H NMR (400MHz, CDCl 3 ,ppm): δ5.41(d,J=9.8Hz,1H),4.28(dd,J=10.9,5.7Hz,1H),4.13–4.04(m,3H),3.95(dd,J=9.8,6.0 Hz,1H),2.33–2.27(m,2H),2.23–2.14(m,3H),2.05–2.01(m,1H),1.87–1.67(m,6H),1.57(d,J=11.6Hz, 2H),144–1.29(m,2H),1.25(t,J=7.1Hz,3H),1.16(t,J=8.0Hz,3H),1.15(s,3H),1.12(t,J=7.7 Hz,3H),1.08–0.90(m,6H),0.88(s,3H),0.76(s,3H); 13 C NMR (100MHz, CDCl 3 , ppm): δ177....
Embodiment 3
[0050] General method for the preparation of embodiment 3 compound I-6~I-8, II-10
[0051]Weigh compounds 4, 8, 12, 14 (10mmol) and dissolve them in 100mL of dichloromethane, add 1mL of acid-binding agent DIPEA and benzoyl chloride (1.6g, 11mmol) respectively, stir and react at room temperature for 2 hours, detect by TLC, and react complete. After the solvent was evaporated under reduced pressure, ethyl acetate and saturated brine were added for extraction, the organic phase was washed three times with saturated brine, the organic phase was dried with anhydrous sodium sulfate, filtered, concentrated, fried, and separated by column chromatography to obtain the corresponding White solid compounds I-6 to I-8 and II-10.
[0052]
[0053] Compound I-6: Yield: 92%; 1 H NMR (400MHz, CDCl 3 , ppm): δ7.75(d, J=7.0Hz, 2H), 7.50(t, J=7.3Hz, 1H), 7.44(t, J=7.2Hz, 1H), 6.10(d, J=8.4Hz ,1H), 4.26–4.20(m,1H),4.13–3.99(m,2H),2.16(d,J=13.2Hz,1H),2.00(dd,J=14.1, 11.6Hz,1H),1.84– 1.59(m,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com